Yi Guo1, Yi Su2, Chun-Hong Shen1, Yi Yang3, Yao Ding1 and Mei-Ping Ding1*
1Department of Neurology, Epilepsy Center, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
2Department of Neurology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
3Department of Neurology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
*Corresponding Author:
Dr. Mei-Ping Ding
Department of Neurology, Epilepsy Center
Second Affiliated Hospital, School of Medicine
Zhejiang University, No. 88, Jiefang Road Hangzhou, 310009, China
Tel: 8657187784750
E-mail: dmp_neurology@hotmail.com
Received Date: January 19, 2017; Accepted Date: February 21, 2017; Published Date: February 23, 2017
Citation: Guo Y, Su Y, Shen CH, et al. 4-Hydroxybenzyl Alcohol Prevents Epileptogenesis As Well As Neuronal Damage in Amygdaloid Kindled Seizures. J Neurol Neurosci. 2017, 8:1. Doi: 10.21767/2171-6625.1000175
Keywords
4-hydroxybenzyl alcohol; Epileptogenesis; Kindling; Neuroprotection
Introduction
Epilepsy, one of the most common neurologic diseases, is characterized by recurrent unprovoked events and its cognitive, psychological and social comorbidities. The etiologies of most cases remain unknown, making it hard to give prophylactic treatment to prevent the development of epilepsy, namely epileptogenesis. The past 20 years have seen the introduction of more than 15 antiepileptic drugs with various mechanisms. Nevertheless, many patients continue to have seizures despite receiving treatment either singly or in combination, mainly because of the failure to block epileptogenesis [1]. Thus, there lies a great potential in drugs targeting at epileptogenesis process [2].
Gastrodia elata (G. elata) Blume has been used since ancient times in China for the treatment of a variety of conditions, including epilepsy [3]. Previous studies have shown the anticonvulsive effects of G. elata and its components in multiple models, including the ferric chloride model in Sprague-Dawley rats [4], kainic acid (KA) injection [5], PTZ injection [6], and amygdala kindled rats [7]. Wang et al. reported that an extract of G. elata shortened the duration of status epilepticus induced by Li-pilocarpine, and decreased neuronal loss in the hippocampus [8]. 4-Hydroxybenzyl alcohol (4-HBA) is one of the bioactive components in G. elata extracts, and it has pharmacological effects that are similar to G. elata [9]. Previous studies have shown that 4-HBA exhibits neuroprotective effects, including antioxidant [10,11], antiapoptosis [12] and antiangiogenic functions [13,14]. Thus, based on the evidence above, we hypothesize that 4-HBA, the main component of G. elata, also have an anticonvulsive effect, making it a potential target for treating epilepsy.
In the model of amygdaloid kindled seizures, repeated administration of initially subconvulsive electrical stimulus eventually results in focal after-discharges that spread and ultimately cause secondarily generalized seizures [15]. Using this model, epileptogenesis can be studied at various defined points during its evolution. Hence, in the present study, we used this model to observe the effect of 4-HBA on epileptogenesis, ictogenesis and neuronal loss.
Materials and Methods
Animals and surgery
Male Sprague-Dawley rats (250-280 g, Grade II, Certificate No. SCXK2003-0001; Experimental Animal Center, Zhejiang Academy of Medical Science, Hangzhou, China) were housed individually and kept at 21 ± 1°C with a 12-h light-dark cycle (lights on from 8:00 am to 8:00 pm). All experiments were carried out in accordance with the ethical guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize both the number of animals used in the study and their suffering in the experiments. Water and food were provided ad libitum. Experiments were performed between 10:00 am and 5:00 pm.
After deep chloral hydrate anesthesia (400 mg/kg, i.p.), rats were mounted on a stereotaxic apparatus (512600; Stoelting, USA). Electrodes were implanted into the right basolateral amygdala (coordinates from the bregma: AP=−2.4 mm, L=−4.8 mm, and V=−8.8 mm). The electrodes were made of twisted stainless steel wires with a diameter of 0.2 mm (A.M. Systems, USA) and Teflon-coated except for 0.5 mm at the tip. The distance between tips was 0.7 - 0.8 mm. The electrodes were connected to a miniature receptacle, which was embedded in the skull with dental cement [16]. Animals were allowed to recover from surgery for 7 days before testing.
Procedure for kindling and threshold measurement
Electroencephalograms (EEGs) of the right amygdala were recorded with a digital amplifier (RM-6240; Chengyi, China). The after-discharge threshold (ADT) of each subject was determined by a constant current stimulator (YC-2; Chengyi, China). An initial current of 50 μA was used, and was subsequently increased stepwise was 20 μA increments. Consecutive trials were separated by at least 30 min. The ADT was defined as the lowest current required to elicit an afterdischarge that lasted at least for 5 s on EEGs [17,18]. All animals were subjected to kindling stimulation with the same current intensity as their own ADT once a day. The daily kindling stimulus consisted of a 1 s train of monophasic, 1-ms square wave pulses at 60 Hz. Animals that exhibited three consecutive stage 5 seizures were considered to be fully kindled [17]. Seizure severity was classified according to a modification of the classification by Racine [19], as follows: (1) facial movements; (2) head nodding; (3) unilateral forelimb clonus; (4) bilateral forelimb clonus and rearing; and (5) bilateral forelimb clonus, rearing and falling. Stages 1-3 were considered to be focal seizures, while stages 4 and 5 were considered to be generalized seizures (GS).
Effect of 4-HBA on kindling acquisition and fully kindled animals
In the first experiment, rats were divided into four groups matched for ADTs. 4-HBA (Sigma) was administered 0.5 h before kindling stimulation once daily at 25, 50 and 100 mg/kg doses i.p.; an equivalent volume of saline was administered in the control group. Animals were stimulated daily at ADT intensity until the control group animals were fully kindled. The seizure stage, after-discharge duration (ADD), cumulative ADD and incidence of GS were measured.
In the second experiment, rats were stimulated until they were fully kindled without any 4-HBA treatment during kindling, and were then divided into four groups as described above. On the next day, the four groups received stimuli with pre-kindling ADT intensity after pretreatment with saline, 25 mg/kg, 50 mg/kg or 100 mg/kg 4-HBA. All rats were pretreated and stimulated daily for 5 consecutive days. The seizure stage, cumulative ADD, incidence of GS and generalized seizure duration (GSD) were measured.
Histology
All animals were sacrificed 2 h after the last stimulation. The rats were deeply anesthetized with chloral hydrate and perfused transcardially with 100 ml of 0.1 mol/l phosphate buffered saline, followed by 100 ml of 4% buffered paraformaldehyde for 10 min. The brain was then carefully removed and fixed at 4°C in 4% paraformaldehyde and 30% buffered sucrose for 3 days. Coronal brain sections of 10-μm thickness were collected for the Nissl protocol. Three tissue sections were randomly selected from each rat and examined independently by two experienced histologists. The numbers of neurons in the hippocampus and parahippocampal cortices, including the bilateral entorhinal and piriform cortices were counted at a 200× magnification in each visual field, from four separate visual fields in each region.
Statistical analysis
A statistical software package, SPSS 13.0 for Windows (SPSS, Inc, Chicago, USA) was used. Data are presented as the mean ± standard error of the mean (S.E.M.). Group differences in kindling acquisition were assessed by a two-way analysis of variance (ANOVA) for repeated measures. Other variables were analyzed with one-way ANOVA when the data were normally distributed and the variances were homogeneous. Otherwise, nonparametric Mann–Whitney U tests were used. Neuronal loss was defined as the percentage of neuron density decrease between day 0 and day 20. For all analyses, the tests were two-sided and P<0.05 was considered to be statistically significant.
Results
The electrodes were found in the right basolateral amygdale in 97 rats, and these animals were included in our study; 73 were investigated for seizure semiology and EEG, while 24 rats were sacrificed by decapitation to collect samples for histology and immunohistochemical analysis. The animals displayed no signs of abdominal cramps or other abnormal behavior after i.p. injections of 4-HBA.
Effect of 4-HBA on kindling acquisition
Experiment 1 included four groups of rats pretreated with 25 mg/kg 4-HBA (n=9), 50 mg/kg 4-HBA (n=9) and 100 mg/kg 4-HBA (n=9) and saline (n=10). Only 4-HBA at the dose of 50 mg/kg delayed seizure stage progression (2-way ANOVA; F=3.162, P=0.037; Figure 1A); both 25 mg/kg and 50 mg/kg doses shortened the corresponding ADD (2-way ANOVA; F=5.532, P=0.003; Figure 1B). However, 100 mg/kg 4-HBA had no effect on either the stepwise progression of kindling or the ADD (Figures 1A and 1B).
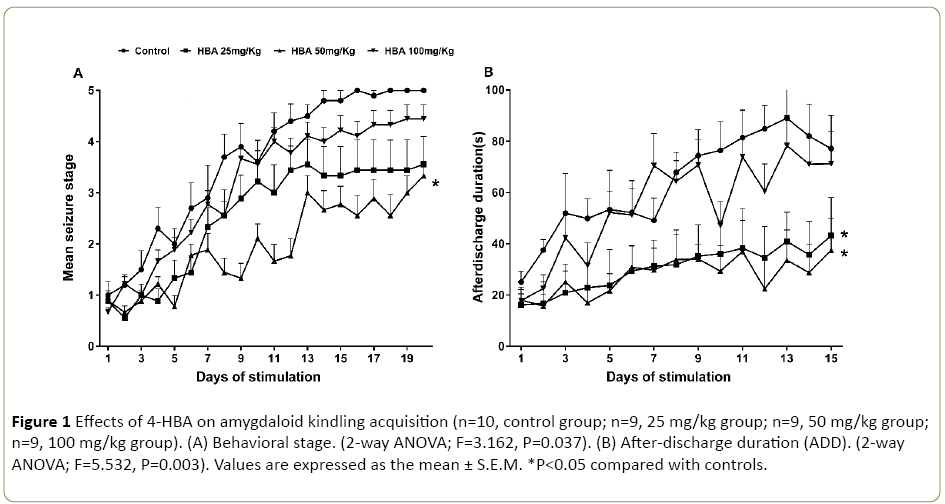
Figure 1: Effects of 4-HBA on amygdaloid kindling acquisition (n=10, control group; n=9, 25 mg/kg group; n=9, 50 mg/kg group; n=9, 100 mg/kg group). (A) Behavioral stage. (2-way ANOVA; F=3.162, P=0.037). (B) After-discharge duration (ADD). (2-way ANOVA; F=5.532, P=0.003). Values are expressed as the mean ± S.E.M. *P<0.05 compared with controls.
To further analyze the stepwise progression of kindling, we calculated the number of stimulations and the cumulative ADD needed to reach each seizure stage or remain at the generalized seizure stages (GS, stages 4-5). 4-HBA at the dose of 50 mg/kg increased the number of stimulations required to reach stages 4-5 (1-way ANOVA; To4 F=2.928, P=0.048; To5 F=2.922, P=0.048; Figure 2A) and prolonged the cumulative ADD to reach stage 5 (1-way ANOVA; cF=3.185, P=0.036; dF=3.309, P=0.028; Figure 2B). 4-HBA at 50 mg/kg reduced the number of stimulations for staying at stages 4-5 (P<0.05; Figure 2C). However, 4-HBA at a dose of 25 mg/kg or 100 mg/kg did not show any significant difference compared with the control group (P>0.05; Figures 2A-2D). In addition, only 4- HBA at the dose of 50 mg/kg decreased the incidence of GS (P<0.05; Figure 2D).
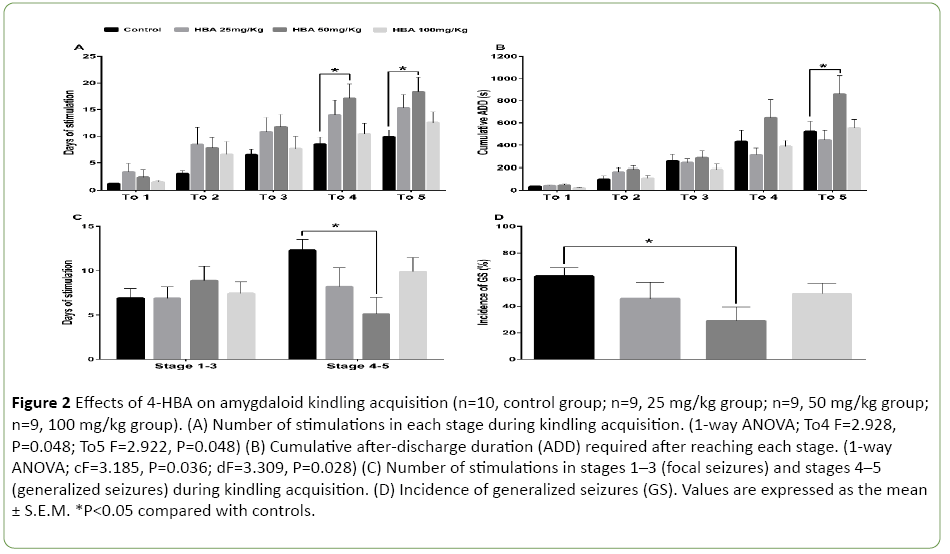
Figure 2: Effects of 4-HBA on amygdaloid kindling acquisition (n=10, control group; n=9, 25 mg/kg group; n=9, 50 mg/kg group; n=9, 100 mg/kg group). (A) Number of stimulations in each stage during kindling acquisition. (1-way ANOVA; To4 F=2.928, P=0.048; To5 F=2.922, P=0.048) (B) Cumulative after-discharge duration (ADD) required after reaching each stage. (1-way ANOVA; cF=3.185, P=0.036; dF=3.309, P=0.028) (C) Number of stimulations in stages 1–3 (focal seizures) and stages 4–5 (generalized seizures) during kindling acquisition. (D) Incidence of generalized seizures (GS). Values are expressed as the mean ± S.E.M. *P<0.05 compared with controls.
Effect of 4-HBA on fully kindled animals
Thirty-six rats were included in this experiment. Treatment with 4-HBA did not decrease the mean seizure stage or the incidence of GS, and did not shorten the cumulative ADD or mean GSD (P>0.05; Figures 3A-3D). No significant differences were found among the 25 mg/kg 4-HBA (n=9), 50 mg/kg 4- HBA (n=9), 100 mg/kg 4-HBA (n=9) and control groups (n=9).
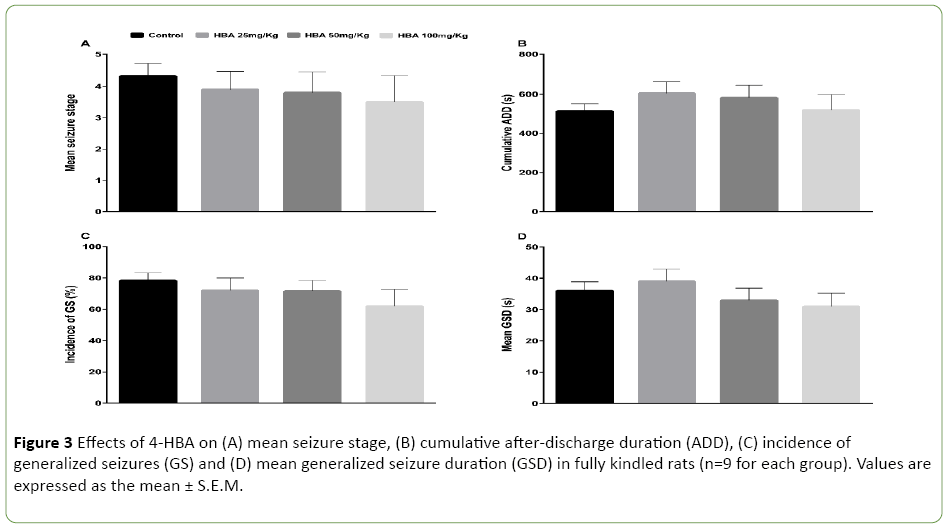
Figure 3: Effects of 4-HBA on (A) mean seizure stage, (B) cumulative after-discharge duration (ADD), (C) incidence of generalized seizures (GS) and (D) mean generalized seizure duration (GSD) in fully kindled rats (n=9 for each group). Values are expressed as the mean ± S.E.M.
Effect of 4-HBA on neuronal loss
In the control group, neuron density significantly decreased in the hippocampus and parahippcampal cortices during kindling. The neuronal loss percentages in the ipsilateral hippocampal CA1, CA3 and DG areas were 25% ± 2.6% (P<0.05), 21.1% ± 1.9% (P<0.05), and 17.1% ± 1.3% (P<0.05), respectively (Figure 4A). The neuronal loss percentages in the ipsilateral piriform cortex, the ipsilateral and the contralateral entorhinal cortices were 13.4% ± 1.6% (P<0.05), 9.8% ± 1.3% (P<0.05) and 10.1% ± 1.8% (P<0.05), respectively (Figures 4A and 4B). After 20 stimulations, 4-HBA at 50 mg/kg attenuated the neuronal loss in the ipsilateral CA1 and the bilateral entorhinal cortex (P<0.05; Figures 4A and 4B), but not in other areas. Neither 25 mg/kg 4-HBA nor 100 mg/kg 4-HBA attenuated the degree of neuronal loss in the bilateral hippocampal and parahippcampal cortices (Figures 4A and 4B).
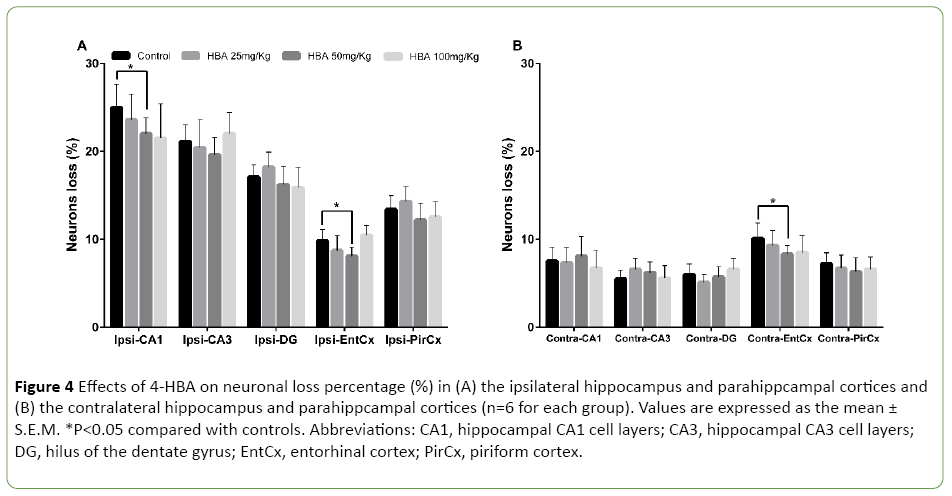
Figure 4: Effects of 4-HBA on neuronal loss percentage (%) in (A) the ipsilateral hippocampus and parahippcampal cortices and (B) the contralateral hippocampus and parahippcampal cortices (n=6 for each group). Values are expressed as the mean ± S.E.M. *P<0.05 compared with controls. Abbreviations: CA1, hippocampal CA1 cell layers; CA3, hippocampal CA3 cell layers; DG, hilus of the dentate gyrus; EntCx, entorhinal cortex; PirCx, piriform cortex.
Discussion
To the best of our knowledge, our study is the first to address the anti-epileptogenic effect of 4-HBA. We demonstrated that 50 mg/kg 4-HBA delayed seizure progression induced by amygdaloid kindling, and shortened the corresponding ADD. Meanwhile, 25 mg/kg 4-HBA only shortened the ADD and 100 mg/kg 4-HBA had no effect on either the progression of kindling or the ADD. This antiepileptogenic function of 4-HBA may be dose-related, which is similar to the neuroprotective effect of 4-HBA on transient focal cerebral ischemia [11]. In that study, the middle dose (50 mg/kg 4-HBA) showed more significantly reduced infarction and neurological deficits than the other doses (25 mg/kg and 100 mg/kg). Various neuroprotective antioxidants of diverse structures and activities also displayed less neuroprotection at higher doses than at lower doses [20,21].
The amygdaloid kindling seizure model enabled us to analyze epileptogenesis in graded stages. At the dose of 50 mg/kg, 4-HBA delayed epileptogenesis from partial to generalized seizures (stages 4–5), and prolonged the cumulative ADD required to reach stage 5. Furthermore, it decreased the incidence of GS, confirming that 4-HBA had an inhibitory effect on secondary generalized discharges. Furthermore, 4-HBA failed to decrease the severity and frequency of generalized seizures for fully kindled rats, which indicated that 4-HBA exerts an anti-epileptogenic effect rather than the anti-ictogenic effect. On the other hand, this phenomena suggests that the anti-epileptogenic effect of 4- HBA is not based on the reduction of seizures. On the contrary, the anti-epileptogenic action of 4-HBA is likely to be related to its neuroprotective effect. Previous studies suggested 4-HBA confers neuroprotection through upregulation of antioxidant protein expression [10], anti-apoptosis [11] and by reducing lipid peroxidation [22]. Protein oxidation and lipid peroxidation are recognized as the contributors to epileptogenesis [23,24]. It is possible that reduced oxidation plays a role in the anti-epileptogenic function of 4-HBA in the late stages of kindling. Further study is needed to confirm this hypothesis.
4-HBA has been known as a neuroprotective agent [10-12,20,22,25,26], and has been shown to decrease neuronal loss in the Li-pilocarpine model [8]. However, there was no evidence that 4-HBA had neuroprotective effects in the amygdaloid kindled model. Therefore, we examined the effect of 4-HBA on neuronal loss in the hippocampus and parahippocampal cortices. Our data was similar to our previous study, in which the neuronal loss in amygdaloid kindled seizures was not widely distributed; there was a mild to moderate neuronal loss in the hippocampal and parahippocampal cortices [27]. In the present study, 50 mg/kg 4-HBA attenuated the neuronal loss in the ipsilateral CA1 area compared with the control group. It has been reported that the hippocampal CA1 area supports epileptogenesis in the kindling model [28]. An inhibition of neural activity of CA1 neurons by local lidocaine injection decreased seizure severity and retarded amygdaloid kindling. Our findings suggest that the neuroprotection of 4-HBA may be involved in the antiepileptogenic action in amygdaloid kindling model.
Parahippocampal structures have dense connections with the amygdala and hippocampus. They play a crucial role in seizure pathways, especially in the secondary generalization of limbic seizures [29]. We found a mild neuronal loss in the entorhinal cortex during kindling; 50 mg/kg 4-HBA significantly attenuated the neuronal loss in the bilateral entorhinal cortex. Our previous study indicated the entorhinal cortex was involved in epileptogenesis in the pilocarpine model [30]. Hence, it is probable that the neuroprotection of 4-HBA in the entorhinal cortex plays a role in the anti-epileptogenic function of 4-HBA in secondary generalized seizures.
Conclusion
We have demonstrated that 4-HBA at 50 mg/kg provides an anti-epileptogenic effect by preventing both after-discharge generation and propagation in amygdaloid kindled seizures. However, 4-HBA had no effect on fully kindled seizures. Moreover, 4-HBA had neuroprotective effects on the ipsilateral CA1 and the bilateral entorhinal neurons. Additional studies are required to address whether the antioxidant properties of 4-HBA plays a role in the anti-epileptogenic function of 4-HBA during the late stage of kindling, which is clinically important to cure epilepsy.
Financial Support and Sponsorship
This work was supported by grants from the National Natural Science Foundation of China [grant number 81471316] and the Traditional Chinese Medicine Program of Zhejiang Province [grant number 2010ZA069].
Conflicts of Interest
None declared.
18527
References
- Mani R, Pollard J, Dichter MA (2011) Human clinical trials in antiepileptogenesis. NeurosciLett 497: 251-256.
- French JA, White HS, Klitgaard H, Holmes GL, Privitera MD, et al. (2013) Development of new treatment approaches for epilepsy: Unmet needs and opportunities. Epilepsia 4: 3-12.
- Ojemann LM, Nelson WL, Shin DS, Rowe AO, Buchanan RA (2006) Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav 8: 376-383.
- Hsieh CL, Chang CH, Chiang SY, Li TC, Tang NY, et al. (2000) Anticonvulsive and free radical scavenging activities of vanillyl alcohol in ferric chloride-induced epileptic seizures in Sprague-Dawley rats. Life Sciences 67: 1185-1195.
- Hsieh CL, Chiang SY, Cheng KS, Lin YH, Tang NY, et al. (2001) Anticonvulsive and free radical scavenging activities of Gastrodiaelata Bl. in kainic acid-treated rats. Am J Chin Med 29: 331-341.
- Ha JH, Lee DU, Lee JT, Kim JS, Yong CS, et al. (2000) 4-Hydroxybenzaldehyde from Gastrodiaelata B1. is active in the antioxidation and GABAergicneuromodulation of the rat brain. Journal Of Ethnopharmacology 73: 329-333.
- Wu HQ, Xie L, Jin XN, Ge Q, Jin H, et al. (1989) The effect of vanillin on the fully amygdala-kindled seizures in the rat. Yao XueXueBao 24: 482-486.
- Wang BG, Yang N, Liao WP, Su T (2009) Neuroprotection of extraction of Gastrodiaelata for status epilepticus induced by li-pilocarpine. Chin J Rehabil Theory Pract 15: 203-205.
- Wu CR, Hsieh MT, Huang SC, Peng WH, Chang YS, et al. Effects of Gastrodiaelata and its active constituents on scopolamine-induced amnesia in rats. PlantaMedica 62: 317-321.
- Yu S, Zhao J, Wang X, Lei S, Wu X, et al. 4-Hydroxybenzyl alcohol confers neuroprotection through up-regulation of antioxidant protein expression. Neurochemical Research 38: 1501-1516.
- Yu SS, Zhao J, Lei SP, Lin XM, Wang LL, et al. (2011) 4-hydroxybenzyl alcohol ameliorates cerebral injury in rats by antioxidant action. Neurochemical Research 36: 339-346.
- Yu SS, Zhao J, Zheng WP, Zhao Y (2010) Neuroprotective effect of 4-hydroxybenzyl alcohol against transient focal cerebral ischemia via anti-apoptosis in rats. Brain Research 1308: 167-175.
- Laschke MW, Vorsterman VOA, Korbel C, Scheuer C, Menger MD (2013) 4-hydroxybenzyl alcohol: A novel inhibitor of tumor angiogenesis and growth. Life Sciences 93: 44-50.
- Laschke MW, Vorsterman VOA, Scheuer C, Menger MD (2011) In vitro and in vivo evaluation of the anti-angiogenic actions of 4-hydroxybenzyl alcohol. Br J Pharmacol 163: 835-844.
- Racine RJ (1972) Modification of seizure activity by electrical stimulation. I. After-discharge threshold. ElectroencephalogrClinNeurophysiol 32: 269-279.
- Jiang Y, Yang Y, Wang S, Ding Y, Guo Y, et al. (2012) Ketogenic diet protects against epileptogenesis as well as neuronal loss in amygdaloid-kindling seizures. NeurosciLett 508: 22-26.
- Yu SS, Zhao J, Zheng WP, Zhao Y (2010) Neuroprotective effect of 4-hydroxybenzyl alcohol against transient focal cerebral ischemia via anti-apoptosis in rats. Brain Res 1308: 167-175.
- Ding Y, Wang S, Jiang Y, Yang Y, Zhang M, et al. (2013) Fructose-1,6-diphosphate protects against epileptogenesis by modifying cation-chloride co-transporters in a model of amygdaloid-kindling temporal epilepticus. Brain Res 1539: 87-94.
- Racine RJ, Gartner JG, Burnham WM (1972) Epileptiform activity and neural plasticity in limbic structures. Brain Research 47: 262-268.
- Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, et al. (2009) Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia 57: 645-656.
- Ley JJ, Belayev L, Saul I, Becker DA, Ginsberg MD (2007) Neuroprotective effect of STAZN, a novel azulenylnitrone antioxidant, in focal cerebral ischemia in rats: dose-response and therapeutic window. Brain Research 1180: 101-110.
- Jung TY, Suh SI, Lee H, Kim IS, Kim HJ, et al. (2007) Protective effects of several components of Gastrodiaelata on lipid peroxidation in gerbil brain homogenates. Phytother Res 21: 960-964.
- Rowles J, Olsen M (2012) Perspectives on the development of antioxidant antiepileptogenic agents. Mini Rev Med Chem 12: 1015-1027.
- Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free RadicBiol Med 62: 121-131.
- Descamps E, Petrault LM, Maurois P, Pages N, Bac P, et al. (2009) Experimental stroke protection induced by 4-hydroxybenzyl alcohol is cancelled by bacitracin. Neuroscience Research 64: 137-142.
- Kam KY, Yu SJ, Jeong N, Hong JH, Jalin AM, et al. (2011) p-Hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2, PDI, and neurotrophic factor genes in a rat model of brain ischemia. Molecules and Cells31: 209-215.
- Jiang Y, Yang Y, Wang S, Ding Y, Guo Y, et al. (2012) Ketogenic diet protects against epileptogenesis as well as neuronal loss in amygdaloid-kindling seizures. Neuroscience Letters 508: 22-26.
- Mirnajafi ZJ, Mortazavi M, Fathollahi Y, Alasvand ZM, Reza PM (2002) Effect of transient hippocampal inhibition on amygdaloid kindled seizures and amygdaloid kindling rate. Brain Research 954: 220-226.
- McIntyre DC, Kelly ME (2000) The parahippocampal cortices and kindling. Ann NY AcadSci 911: 343-354.
- Guo Y, Gao F, Wang S, Ding Y, Zhang H, et al. (2009) In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: An 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience 162: 972-979.









