Kairavi Bhardwaj1*, Shiv Kumar Sharma1, Pandey AK2 and Vaishali Upadhyaya3
1Vivekananda Polyclinic and Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
2Department of Neurology, Vivekananda Polyclinic and Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
3Department of Radiodiagnosis, Vivekananda Polyclinic and Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
Corresponding Author:
Kairavi Bhardwaj
DNB Medicine, Vivekananda Polyclinic and Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
Tel: +91-7388905353
E-mail: kairavib@gmail.com
Received date: July 21, 2016; Accepted date: August 12, 2016; Published date: August 16, 2016
Citation: Bhardwaj K, Sharma SK, Pandey AK, et al. A Case of Limbic Encephalitis: Antibody LGI1 Associated Encephalitis. J Neurol Neurosci. 2016, 7:4. doi: 10.21767/2171-6625.1000137
Keywords
Limbic Encephalitis (LE); Voltage Gated Potassium Channels (VGKC); LGI1 (Leucine rich Glioma inactivated 1); Autoimmune
Introduction
Limbic Encephalitis (LE) is an uncommon neurological disorder encountered rarely in day to day clinical practice. In Indian settings, a clear statistical data on its frequency of occurrence is not available but few case reports have been published here and there. It is characterized by a variety of clinical presentations and lack of symptom specificity including seizures, memory problems, irritability, depression, confusion and dementia leading to wide range of differential diagnosis [1]. Viral infections, inflammatory or autoimmune disorders (lupus, Sjogren’s, Hashimoto thyroiditis and CNS vasculitis), toxic and metabolic encephalopathy, paraneoplastic syndromes and autoimmunity become the possible clinical etiologies. Classically, limbic encephalitis was mostly considered a paraneoplastic disorder [2,3] but many new antibodies are being discovered in patients of Limbic Encephalitis which are not associated with tumours, thereby arising possibility of autoimmune basis to this disease [1,4].
Limbic encephalitis is a rare disorder affecting the medial temporal lobe of the brain. It was first described by Breirly et al. in 1960s when they reported 3 cases of sub-acute encephalitis involving the limbic area [5]. In 1968 Corsellis et al. coined the term “limbic encephalitis” and also established the relationship between limbic encephalitis and systemic cancer [2]. Many neuronal antibodies have been associated with LE. These can be directed either against intracellular antigens (classic paraneoplastic), including Hu, CV2/CRMP5, Ma2 and amphiphysin or against cell membrane antigens, including VGKC (voltage-gated potassium channels), N-methyl- D-aspartate receptor (NMDA) and Glutamic Acid Decarboxylase receptors (GAD) expressed in the neuropil of hippocampus and cerebellum [6,7]. The former category is related to cancer (paraneoplastic limbic encephalitis), showing limited response to immunomodulatory therapy whereas the latter category is autoimmune in nature being less frequently associated with tumours and responding significantly better to immunomodulatory therapy. It has also been recognized that some patients presenting with limbic encephalitis with negative antibody screen in serum and CSF show full recovery after treatment with steroids or immunomodulatory therapy indicating an autoimmune etiology [8,9].
Here we report an interesting case of a 65 year old woman who presented with memory loss, repetitive episodes of focal seizures without loss of consciousness, with behavioural abnormalities. Imaging suggested a diagnosis of limbic encephalitis which was further confirmed with antibody LGI1 being positive. Further imaging to look for etiology revealed no evidence of any mass lesion, tumour or secondaries anywhere in the body. This case presented here is unique and scientifically relevant, as it intends to raise awareness of Limbic Encephalitis associated with autoimmune antibody, as a potentially reversible cause of a medical emergency.
Case Report
A 65 year old lady presented to the emergency department of this hospital in April 2016 with presentation of 3 episodes of generalized tonic clonic seizures one after the other in a span of half an hour leaving the patient in an unconscious state on arrival to hospital.
Since last 20 days, patient had been getting repeated attacks of focal seizures which used to begin in the form of localized twitching over face progressing over any one half of the face along with repeated jerky movement of head lasting for 5-10 seconds at once, followed by a state of postictal confusion and drowsiness which used to last for another 20 to 30 minutes. Sometimes the focal seizures used to involve one hand but were mostly noticed over face. These episodes used to initially occur 3 to 4 times in a day but their frequency gradually increased in due course of time so much so that on the day of presentation to this hospital, she was getting these attacks after every 20 minutes approximately with sustained postictal confusion and drowsiness until the next attack happened. Along with these recurrent seizure episodes, patient also developed memory impairment especially with disturbances in recent memory and inability to remember family members but with intact past memory. She had behavioural impairment in the form of speaking inappropriate words at unusual times, along with laughing and crying abruptly at odd hours. She used to forget if she had eaten food or if she had gone to toilet. She also sometimes used to start removing her clothes inappropriately at odd times.
For last 9 months, she used to also complain of burning sensation over both feet mainly over the sole of feet at all times of the day with no associated numbness or needle like sensations. This pointed to peripheral neuropathy with which she was suffering from in the last year with underlying cause not known. Patient had undergone cholecystectomy operation 8 years back in a private hospital due to cholelithiasis. No records of the surgery were available.
On admission, she was given loading dose of antiepileptic lorazepam and phenytoin to control seizure activity along with symptomatic treatment with close monitoring of vitals. She regained consciousness the next day but the seizures in the form of focal and complex partial seizures continued to occur at close intervals. Also in the intervening time she continued to remain confused and drowsy. Her MMSE (Mini Mental State Examination) at that time was 18/30 and behavioural abnormalities also persisted.
Serum sodium was found to be low initially (108 meq/ml) which was corrected with 3% NS infusion along with dietary modifications. It rose to normal levels (132 meq/ml) in 2 days and continued to remain in normal range thereafter.
Contrast enhanced MRI Brain was done which revealed hyper intense signal in T2W and T2W FLAIR (Fluid Attenuated inversion recovery) images in the right hippocampus (Figure 1). No restricted diffusion or contrast enhancement was seen. Differentials included limbic encephalitis, herpetic encephalitis and sub-acute infarct. Considering the clinical profile of the patient, the possibility of limbic encephalitis was suggested. CSF was done which showed no significant abnormality. EEG was done which revealed no significant abnormality (Figure 2).
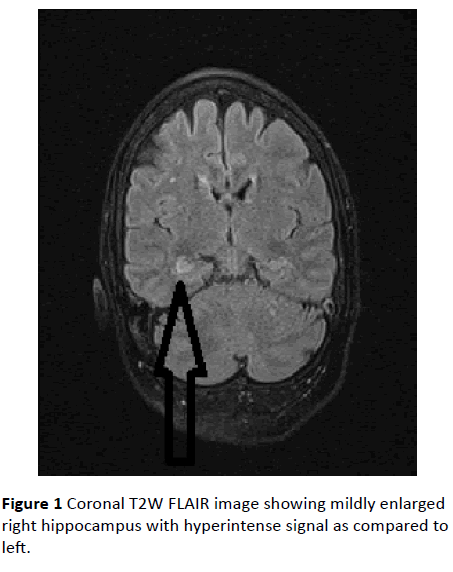
Figure 1: Coronal T2W FLAIR image showing mildly enlarged right hippocampus with hyperintense signal as compared to left.
Figure 2: EEG showing normal awake rhythm.
Further to look for tumour, HRCT Thorax (High resolution computed tomography) and CT Abdomen were done where no evidence of any tumour growth was found (Figures 3 and 4). Autoimmune panel revealed Positive Antibody LGI1 which is part of VGKC. Patient was then started with immunomodulatory therapy in the form of high dose steroids (Injection Methylprednisolone 1 gm IV OD) along with sodium valproate and clonazepam. The frequency of seizures dropped down drastically and no more episodes were witnessed after 48 hours of starting this treatment. Even the memory disturbances and behavioural abnormalities got minimized significantly (MMSE rose to 24/30) and patient’s general condition improved considerably. Steroids were later changed to oral alternatives and patient had been keeping well thereafter.
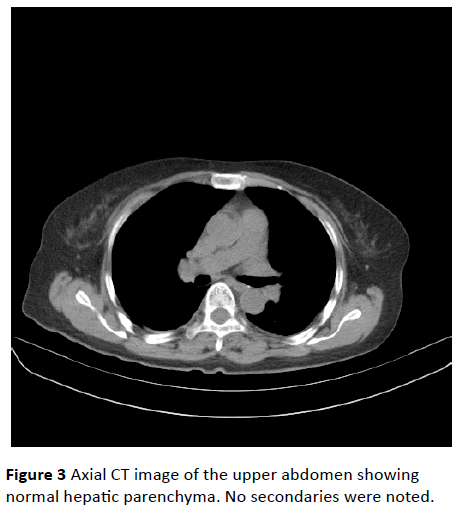
Figure 3: Axial CT image of the upper abdomen showing normal hepatic parenchyma. No secondaries were noted.
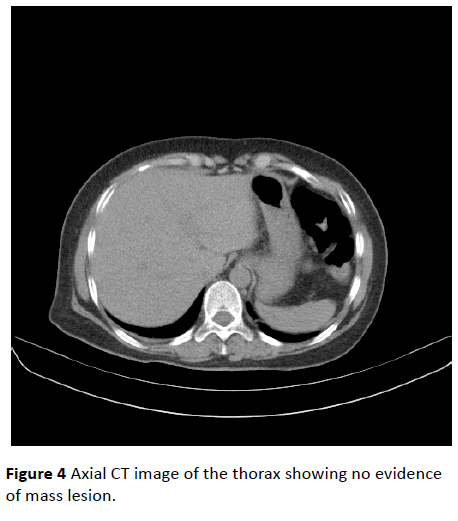
Figure 4: Axial CT image of the thorax showing no evidence of mass lesion.
After a week of discharge, PET-CT scan (Positron Emission Tomography) was done in order to completely rule out possibility of any tumour in the body, and it revealed no significant abnormality. Patient had responded well to immunomodulatory treatment and remains in follow up since then.
Discussion
Limbic encephalitis is a diagnostic challenge. It classically presents with a constellation of symptoms including memory disturbances, complex partial seizures along with behavioural abnormalities and sleep disturbances [2]. Etiology wise, autoimmune limbic encephalitis can either be associated with antibodies to intracellular neuronal antigens, which include all previously known paraneoplastic antigens, and the other associated with antibodies to cell membrane antigens, including the VGKC, NMDA receptor, GAD which have no evidence of any tumour in body [10].
The paraneoplastic ones are difficult to treat and may respond minimally only if the primary tumour source is removed but the autoimmune ones without any tumour presence in body respond considerably more to immunomodulatory treatment and their prognosis is comparatively better. Limbic encephalitis which is antibody LGI1 associated (part of VGKC) is many a times linked with development of hyponatremia. In a study conducted in the United States, 60% of the patients experienced hyponatremia which can be related to syndrome of inappropriate secretion of Antidiuretic Hormone by the LGI1 expression in the hypothalamus and the kidney [11].
In the above mentioned patient, she presented with a history of recurrent seizures along with memory disturbances and behavioural abnormalities. She had sleep disturbances and frequent vacant stare looks. She also had a past history of peripheral neuropathy in the form of burning sensation in both soles of feet. She was detected with hyponatremia at the time of admission even before anti-epileptic drugs were started. Hyponatremia is known to occur commonly in people with anti LGI1 associated limbic encephalitis [11]. However, hyponatremia is a nonspecific sign, because it is also caused by other medical conditions such as decreased salt intake, salt loss by vomiting and diarrhoea, or medical disorders including hypothyroidism or syndrome of inappropriate antidiuretic hormone secretion. Cognitive impairment and confusion, which are cardinal manifestations of LE, are also found in patients with hyponatremia. In our patient the symptoms of seizures along with memory impairment and behavioural abnormalities persisted even after correction of sodium levels to normal. So an alternative diagnosis had to be considered. CSF study was found to be normal ruling out any infective (viral, bacterial, tubercular) cause of her symptoms. MRI Brain revealed non enhancing altered signal intensity in the right hippocampus without any diffusion restriction and suggested the possibility of LE. Keeping this in mind, further work up was done to confirm the provisional diagnosis and to know its etiology, so that effective treatment could be planned.
To rule out paraneoplastic etiology, CT whole abdomen, HRCT thorax and PET CT of whole body was done which showed no evidence of any tumour, mass lesion or secondaries anywhere in body. Results of autoimmune workup revealed positive LGI1 antibody which is part of VGKC. That confirmed our diagnosis. Patient was then immediately started on immunomodulatory treatment in the form of Injection, Methyl Prednisolone 1 gm IV OD along with sodium valproate and clonazepam. Subsequently, patient showed major signs of recovery in the form of reduction in number of seizure episodes and memory improvement. Behavioural disturbances settled down and patient became fully oriented to time place and person. Henceforth, a final diagnosis of limbic encephalitis was made which was autoimmune in nature associated with antibody LGI1.
Anti-LGI1 LE usually involves the medial temporal area, which causes memory dysfunction and seizures. This disorder was recently identified as autoimmune encephalitis, which has distinctive clinical features, such as limbic dysfunction, seizures, and occasional hyponatremia. In Anti-LGI1 LE, MRI may show signal changes in medial temporal lobes and basal ganglia. However, a number of patients do not show abnormalities on MRI or PET, and evidence of inflammation, including pleocytosis or protein elevation, can be absent from CSF. Accordingly, typical clinical manifestations are necessary for prompt antibody diagnoses.
Corticosteroids, intravenous immunoglobulin and plasma exchange are most frequently used as therapeutic options. Other immune-suppressive agents like cyclophosphamide and rituximab can also be utilized as a therapeutic option [9,12]. A study conducted by Saidha et al. [13] also shows promising results with decrease in seizure frequency and improvement in behaviour memory testing in these patients with use of mycophenolate mofetil. Development of these therapeutic options highlights the need for early detection and aggressive management for patients with autoimmune limbic encephalitis. However in cases where evidence of tumour is found, efforts to remove it or treat it by means of surgery, radiotherapy or chemotherapy can be sought after. If this disease process is considered early, diagnosed promptly and treated appropriately, it can be reversed and the patient can be restored to their premorbid state.
Conclusion
Limbic encephalitis is a rare diagnosis but if detected timely and with underlying cause detected correctly, proper effective treatment is available which can remarkably benefit a patient. Classically thought to be of autoimmune etiology, more and more cases are being diagnosed having a paraneoplastic source as well. Henceforth, a thorough investigative work up is required to look for paraneoplastic evidence to disease. It is important that we consider autoimmune limbic encephalitis in our differential diagnosis in adults with encephalopathy, particularly if psychiatric symptoms are seen. Therapeutic responsiveness of this condition reiterates the importance of diagnosing a reversible neurologic pathology. With timely intervention, clinicians may be able to avoid permanent cognitive and behavioural damage.
11215
References
- Tuzun E, Dalmau J (2007) Limbic encephalitis and variants: Classification, diagnosis and treatment. Neurologist 13: 261–271.
- Corsellis JA, Goldberg GJ, Norton AR (1968) "Limbic encephalitis" and its association with carcinoma. Brain 91: 481–496
- Barkheit AM, Kennedy PG, Behan PO (1990) Paraneoplastic limbic encephalitis: clinico-pathological correlations. J NeurolNeurosurg Psychiatry 53:1084-1088.
- Graus F, Saiz A (2005) Limbic encephalitis: A probably under-recognized syndrome. Neurologia 20: 24–30.
- Brierley JB, Corsellis JAN, Hierons R (1960) Subacute encephalitis of later adult life. Mainly affecting the limbic areas. Brain 83: 357–368.
- Anderson NE, Barber PA (2008) Limbic encephalitis - a review. J ClinNeurosci 15: 961–971.
- Florance NR, Davis RL, Lam C, Szperka C, Zhou L, et al. (2009) Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 66: 11–18.
- Urbach H, Soeder BM, Jeub M, Klockgether T, Meyer B, et al. (2006) Serial MRI of limbic encephalitis. Neuroradiology 48: 380–386.
- Bataller L, Kleopa KA, Wu GF, Rossi JE, Rosenfeld MR, et al. (2007) Autoimmune limbic encephalitis in 39 patients: immunophenotypes and outcomes. J NeurolNeurosurg Psychiatry 78: 381–385.
- Darnell RB, Posner JB (2003) Paraneoplastic syndromes involving the nervous system. N Engl J Med 349: 1543-1554.
- Lai M, Huijbers MG, Lancaster E (2010) Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels:a case series. Lancet Neurol 9: 776-785.
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, et al. (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7: 1091–1098.
- Saidha S, Murphy S, Ronayne A, McCarthy P, Hennessy MJ, et al. (2010) Treatment of anti-glutamic acid decarboxylase antibody-associated limbic encephalitis with mycophenolatemofetil. J Neurol 257:1035–1038.









