Raquel Duarte1,2, Andrea Afonso3,4,5,6, Carina Ferreira3,4,7, Susana Roque3,4, Olga Vasconcelos8, Rui Sarmento-Castro3,4,8, Margarida Tavares9, Cátia Caldas9, Rena Greenwald10, Konstantin Lyashchenko10, Margarida Correia-Neves3,4*, Claudia Nobrega3,4
1Serviço de Pneumologia, Centro de Diagnóstico Pneumológico de Vila Nova de Gaia, Vila Nova de Gaia, Portugal
2Serviço de Epidemiologia, Faculdade de Medicina da Universidade do Porto, Porto, Portugal
3Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Braga, Portugal
4ICVS/3B’s-PT Government Associate Laboratory, Braga/Guimarães, Portugal
5Laboratório de Saúde Pública, Centro de Saúde de Bragança, Bragança, Portugal
6Laboratory of Microbiology and Immunology of Infection, Institute for Molecular and Cell Biology (IBMC), Porto, Portugal.
7Serviço de Patologia Clínica, Centro Hospitalar do Alto Ave, Guimarães, Portugal
8Serviço de Doenças Infecciosas, Hospital Joaquim Urbano, Porto, Portugal
9Serviço de Doenças Infecciosas, Hospital de São João, Porto, Portugal.
10Chembio Diagnostic Systems, Inc., Medford, New York, USA.
*Corresponding Author:
Margarida Correia-Neves
Life and Health Sciences Research Institute
School of Health Sciences, University of Minho
Campus Gualtar, 4710-057 Braga, Portugal
Tel:(+351) 253 604 807
Fax: (+351) 253 604 847
E-mail: mcorreianeves@ecsaude.uminho.pt.
Key words
Tuberculosis, diagnosis, serological tests
Introduction
Tuberculosis (TB) represents an important Public Health problem worldwide which was declared as an emergency by WHO in 2008 [1]. In Portugal, TB incidence is decreasing throughout the last 15 years (from 66.4 to 24.1 per 100,000 individuals from 1985 to 2010). However, it still remains the highest among other Western European countries [2].
Accurate TB diagnosis requires laboratory confirmation. Mycobacterial culture represents the only definitive diagnostic method. However, this approach lacks sensitivity for paucibacillary clinical samples. It also requires invasive procedures for the diagnosis of extra-pulmonary TB. The delay of the culture results, which can reach up to 8 weeks, is an additional disadvantage. Sputum smear microscopy is faster but requires multiple patient visits, and the sensitivity is lower than the culture method particularly in immunocompromised patients [3]. The development of novel serodiagnostic tools to improve TB diagnosis is therefore considered one of the top priorities in TB management [4]. Since the use of Mycobacterium tuberculosis specific antigens renders serodiagnostic tests highly specific [5], we have evaluated in the present work the Chembio TB STAT-PAK II kit [6] and the multiantigen print immunoassay (MAPIA) [7] to determine whether these tests could complement the current TB diagnostic methods in Portugal.
Material and Methods
We performed a multicentre case-control study with individuals followed up in four medical institutions (a TB outpatient clinic, two Hospitals, and a Community Health Centre). All individuals involved in the study were adults Portugal residents. The study was approved by the Hospital de S. João, Porto, Ethics Board. All individuals have signed an informed consent; data was handled anonymously.
Five main groups were included in the analysis (Table 1): 1) Healthy control group that included persons with no history of TB, no known close contact with patients with active TB, resident in a TB free area for the previous 5 years and Quantiferon Gold TB Gold (IGRA) negative; 2) Patients with non-TB lung pathology, IGRA negative, no history of TB or known close contact with patients with active TB; 3) human immunodeficiency virus (HIV)-negative patients with active TB confirmed by M. tuberculosis culture. This group was further divided depending on the duration of the antibacillary therapy as: less than 2 weeks; 1 to 2 months; more than 6 months; 4) HIV-co-infected TB patients with active TB confirmed by M. tuberculosis culture; 5) Recently exposed individuals with probable latent TB infection that exhibit no symptoms of TB, with no previous history of TB or recent contact with a smear positive TB patient and IGRA positive.
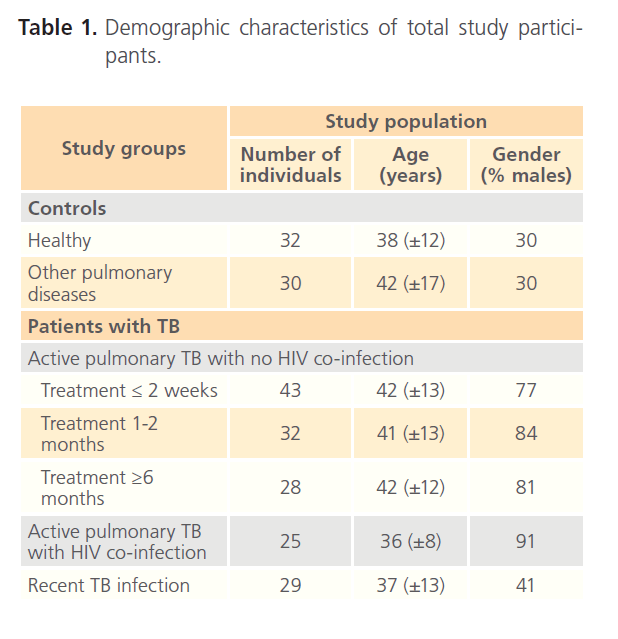
Table 1: Demographic characteristics of total study participants.
Sera were tested for the presence of specific IgG antibodies using TB STAT-PAK II and MAPIA (both from Chembio Diagnostic Systems) performed in accordance with manufacturer’s instructions, as previously described [8]. TB STAT-PAK II is a ready-to-use disposable plastic device based on colored latex lateral-flow technology containing a cocktail of selected M. tuberculosis antigens including ESAT-6, CFP10 and MPB83. Results were read by two independent operators. Any visible band in the test area, in addition to the control line, was considered a positive result [6]. The MAPIA test entails the application of antigens on nitrocellulose membranes, by microaerolization, followed by antibody detection using standard chromogenic immunodevelopment. In this assay, sera was tested against a panel of M. tuberculosis recombinant antigens (ESAT-6, CFP10, MPT59, MPT64, MPT70, MTB83, Acr1, 38 kDa, ESAR6/CFP10 and Acr1/MPB83) [7].
The specificity of each serodiagnostic test was evaluated on the control groups and is the ratio of the number or cases that were negative using the serodiagnostic test with the number of cases that were negative by M. tuberculosis culture. The sensibility of the tests was calculated as the ratio of the number of cases that were positive using the serodiagnostic test with the total number of cases that were positive by M. tuberculosis culture.
Results
The two serodiagnostic tests, TB STAT-PAK II and MAPIA, showed high diagnostic specificities when tested on healthy individuals and on patients with other pulmonary diseases (Table 2). The TB STAT-PAK II sensitivity, compared to the gold standard microbiology culture, varied within the study groups from 59% in HIV-negative TB patients at 1 to 2 months of treatment to 16% in TB/HIV patients. The MAPIA test was more sensitive in patients on less than 2 weeks of treatment in comparison to the TB STAT-PAK II (54% vs. 27%) and less sensitive for TB/HIV patients (8% vs. 16%). No, or very low antibody levels, were detected in recently infected individuals (14% sensitivity by TB STAT-PAK II and 0% by MAPIA).
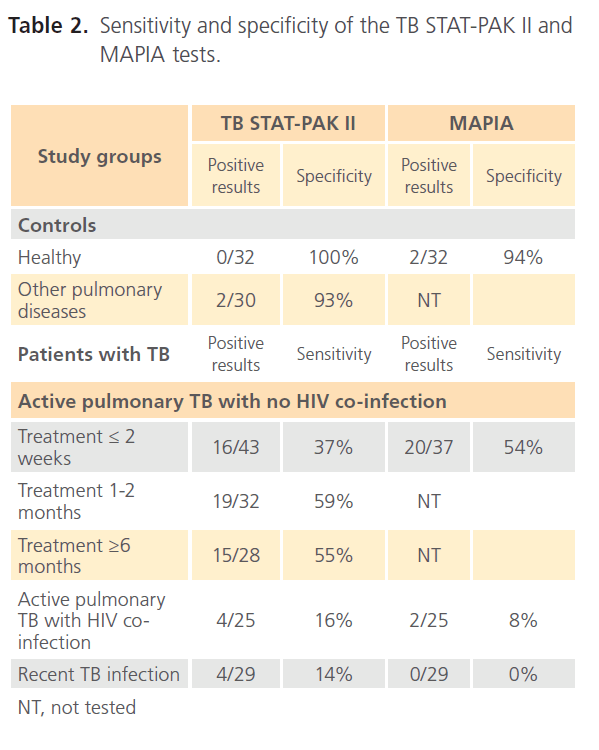
Table 2: Sensitivity and specificity of the TB STAT-PAK II and MAPIA tests.
Despite sputum smear microscopy (upon Ziehl-Neelsen staining) is a hallmark diagnostic test for TB, it only detected about 76% of the cases that were positive by culture. However, when testing the same patients with the serodiagnostic tests an additional 6% and 13% of positive cases were detected using the TB-STAT PAK II and MAPIA, respectively, for HIVnegative TB patients (Figure 1).
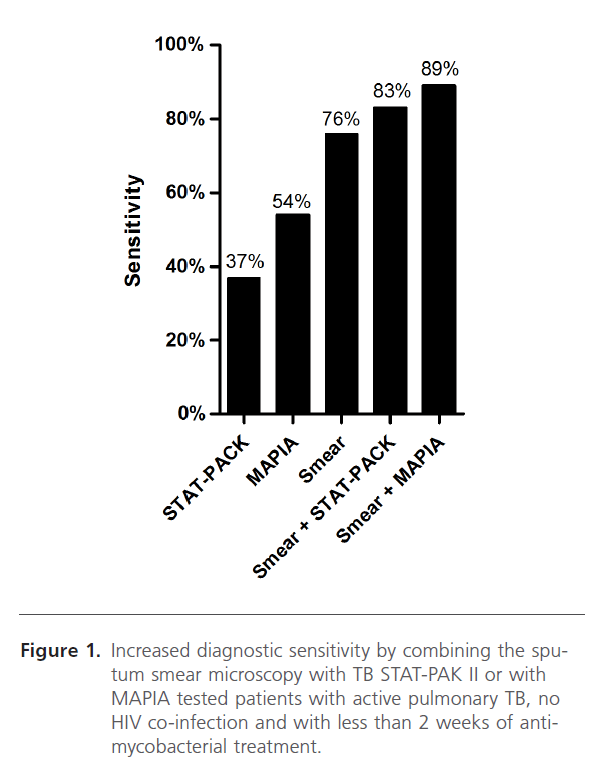
Figure 1: Increased diagnostic sensitivity by combining the sputum smear microscopy with TB STAT-PAK II or with MAPIA tested patients with active pulmonary TB, no HIV co-infection and with less than 2 weeks of antimycobacterial treatment.
Discussion
This study is the first to be performed on the usefulness of serodiagnostic tests for the rapid detection of TB in a Portuguese population. It shows that fast serodiagnostic tests, when combined with sputum smear microscopy, may improve detection of active TB. This may be of particular importance in Portugal and other countries where reducing TB incidence depends mostly on the ability to perform early diagnosis. The two serodiagnostic tests evaluated showed high specificity but neither of the tests alone provided satisfactory sensitivity. For HIV-negative TB patients, sensitivity ranged between 16%-59% using the TB STAT-PAK II test, depending on the duration of anti-mycobacterial therapy. In fact Azzurri et al. have shown that anti-bacillary treatment affect the levels of specific antibodies providing evidence of serological markers of TB and response to treatment [9]. Our data thus suggests that the TB STAT-PAK II test may be a helpful approach to monitor the effectiveness of TB therapy. Unfortunately, the vast majority of the recently infected asymptomatic contacts were negative for both methods which sustain that serodiagnosis is most suitable to identify active TB patients [10].
Despite the low sensitivity of these serodiagnostic tests, when combined with sputum smear microscopy, the most commonly used point-of-care diagnostic procedure, the overall sensitivity increases to 83% and 89% for TB STAT-PACK II and MAPIA, respectively. Therefore, if serodiagnosis becomes a standard procedure in sputum smear negative TB-suspected individuals, up to 13% additional TB cases might be identified shortly after suspicion and before obtaining microbiology culture results, presumably leading to a decrease in the transmission of the disease.
Further efforts to improve serological tests for TB are urgently needed, since the benefits of emerging rapid point-of-care diagnostics tools are evident. The potential of this approach has been recently demonstrated for wildlife species [8]. Future studies, in addition to the conventional evaluation of single test sensitivity and specificity, should develop improved diagnostic algorithms and determine the relative contributions of newly developed assays to the overall clinical decision making process.
Acknowledgment
The authors are grateful to Peter Andersen, James McNair, and Raymond Houghton for kindly providing recombinant antigens used in this study. The project was supported by SBIR Grant Number AI043781 from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
156
References
- WHO (2008) Global tuberculosis control: surveillance, planning, financing(“WHO/HTM/TB/2008.393). World Health Organization
- Antunes F (2010) Ponto da situaçãoepidemiológica e de desempenho. Programanacional de luta contra a tuberculose. DirecçãoGeral da Saúde. https://www.dgs.pt/upload/membro.id/ficheiros/i006111.pdf Access 10.Mar.2011:
- Finch D, Beaty CD (1997) The utility of a single sputum specimen in the diagnosis of tuberculosis. Comparison between HIV-infected and non-HIV-infected patients. Chest 111:1174-1179.
- WHO (2007) Tuberculosis Diagnostics Workshop: Product Development Guidelines. World Health Organization 1.
- Steingart KR, Dendukuri N, Henry M, Schiller I, Nahid P et al. (2009) Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol 16:260-276.
- Lyashchenko KP, Greenwald R, Esfandiari J, Greenwald D, Nacy CA et al. (2007) PrimaTB STAT-PAK assay, a novel, rapid lateral-flow test for tuberculosis in nonhuman primates. Clin Vaccine Immunol 14:1158- 1164.
- Lyashchenko KP, Singh M, Colangeli R, Gennaro ML (2000) A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J Immunol Methods 242:91-100.
- Lyashchenko KP, Greenwald R, Esfandiari J, Chambers MA, Vicente J et al. (2008) Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet Microbiol 132:283-292.
- Azzurri A, Kanaujia GV, Sow OY, Bah B, Diallo A et al. (2006) Serological markers of pulmonary tuberculosis and of response to anti-tuberculosis treatment in a patient population in Guinea. Int J ImmunopatholPharmacol 19:199-208.
- Pai M, Kalantri S, Dheda K (2006) New tools and emerging technologies for the diagnosis of tuberculosis: part I. Latent tuberculosis. Expert Rev MolDiagn 6:413-422








