Keywords
Biobank; Europe; Publications
Introduction
The collection of tissue samples can be characterized as a core element in the progression of medical research through the centuries. Although there is substantial disagreement among scholars about the precise definition of a biobank, there is a widespread view of them as repositories that assemble, store and manage human specimens and related data. These biorepositories have existed in some form for over 60 years and a recent surge in numbers has been noticed [1,2], which coincides with success in sequencing the human genome in 2003, the subsequent explosion of new bioinformatic technologies and the vision of improved health through genomic medicine [1,3-5]. As a result, the degree of complexity, specialization, and diversity that exists among modern human research biobanks has led to difficulties regarding the co-operative relations of these kinds of organizations with researchers, investigators and corporations that fund and manage these entities [6]. Moreover, aside member catalogs found in networks of biobanks, few surveys have been conducted in a national or international scale regarding the summarization and further classification of biobanks. We hope to address this issue on one level, by gathering information about the numbers, types, orientation and methods followed by biobanks in Europe, specifically about those included in 2 large networks; Biobanking and BioMolecular resources Research Infrastructure (BBMRI) [7] and EuroBioBank [8]. Simultaneously, we seek to provide a short review describing the key aspects of a biobank as well as categorizing them, taking into account published research work in which they had a significant contribution. In addition, we attempt to extract useful results about any correlation between the different factors mentioned.
Material and Methods
Our work included collecting data from 2 networks of European biobanks (BBMRI and EuroBioBank). We proceeded by organizing these data in 2 wide sectors: biobank’s characteristics and biobank’s scientific outreach. The first category (biobank’s characteristics) comprises of the following: type, research focus, type of collected biological samples and location. For the purpose of our study, the second category (biobank’s scientific outreach) is considered equivalent to the number of recent publications of research teams which have used the biological resources of the biobanks. Furthermore, in order to offer accurate results, we used this index of scientific output as a way to choose our sample from the over 350 biobanks present in the databases of the above organizations. Including only members with published papers, a number of 56 biobanks constitutes our sample.
It should be noted that following the above method has resulted in a sample, where numerous countries with biobanking activity are not represented. Examples of other European countries with biobanking organizations or networks include Sweden (Medical Biobank at Umeå University, Malmö Microbiology Biobank etc.), Norway (Janus Serumbank etc), Denmark (Diet, Cancer and Health Biobank etc.), Iceland (Tissue Archives at the Pathology Department of the University Hospital in Reykjavik etc.), Switzerland (biobank-suisse network), Greece (Panhellenic Biobank of Neurological Disorders) and others. We suggest the conduction of additional studies in order to apprehend the extent of biobanking organizations in Europe and worldwide.
As a final step to our method, we cross-examined the elements of the 2 sectors producing 2 pairs for comparison and determined the degree of correlation (if any) between them.
Characterization of Biobanks
Types of biobanks
Biobanks can be classified by many and heterogeneous criteria [1,9-11]. For example, the ownership of a biobank varies greatly (academic institutions, hospitals, public, private, or in partnership across sector boundaries). According to Kang et al. [5], 60% of the world’s biobanks are sponsored by governments or national corporations, while approximately 17% of them are financially supported by universities, hospitals [12] and non-profit organizations. Specifically for Europe, data concentrated during EUROGENBANK project [9], demonstrate that most institutions storing human samples are within hospitals or health institutes (public or private not-for-profit). Another way of characterizing these kinds of organizations is the purpose/intended use of specimens (research, forensics, transplantation, source for therapeutics or diagnostics). Moreover, the types of collected tissue, which will be examined in the corresponding sector as well as the size of the donors’ group, are factors which should be taken into account. On the other hand, the purpose of this study is to categorize biobanks using a more general criterion, referring to the origins of the group of participants/volunteer group; whether the individual institution is a population-based (such as all newborns, adults, or pregnant women) [13] or a clinical/ disease-oriented type of biobank (including only those with a specific disease) [6,12,14]. The aforementioned classification criteria are depicted in Figure 1.
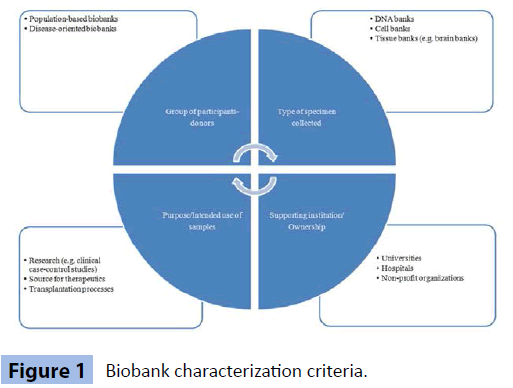
Figure 1: Biobank characterization criteria.
From the 56 biobanks studied, 41 of them listed definite information. Specifically, 37 of them are characterized as clinical biobanks and only 4 of them as population-based. This is expressed as a percentage of 90%, an outcome which we interpret as expected, due to the more complicated structure of projects seeking to collect samples from a large portion of a population.
Research focus of biobanks
The disease spectrum of a biobank’s research activities is often quite diverse. Innovations and technological advancements in the field of health sciences have contributed to the acceleration of biological specimens’ analysis methods. Therefore, scientists are able to produce results concerning a wide range of physiological conditions and disorders, confined to distinguished cells, or even regarding whole organ systems. Although, there isn’t a universal type of categorization regarding every individual biobank’s scientific interest, BBMRI has set up 17 disease categories based on the International Classification of Diseases (ICD) [15], which cover the majority of the clinical phenotypes studied. We adjusted the data gathered from the EuroBioBank database to these categories by thoroughly examining the information provided by each member. In addition, it is not obligatory for every biobank to belong to a single category as the ones with multiple fields of disease research are the majority. It should also be noted that biobanks stating that they are not concerned with a particular set of diseases, were put into the 18th category, named “General/Other”.
Studying the Figure 2 allocation, we need to acknowledge the increased focus on neoplasms (both malignant and benign) as well as research attempts concerning disorders of the central and peripheral nervous system. Other fields of interest seem to be diseases of the endocrine glands and diabetes, heart and circulatory health issues as well as soft tissue deformations. Finally, anaemias and congenital malformations research projects are also noteworthy fields where researcher’s efforts and biobanks supply of samples overlap.
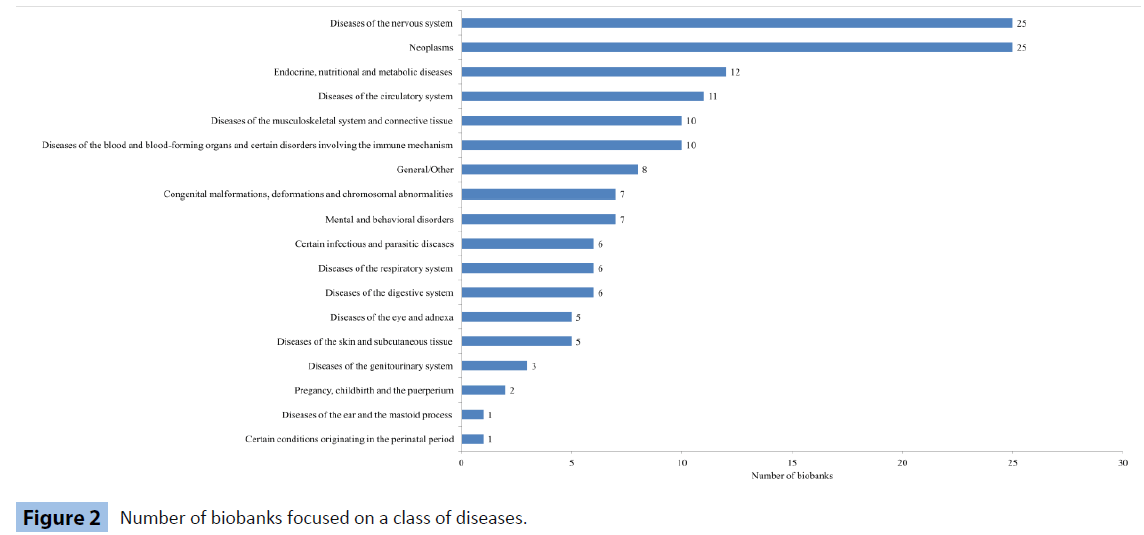
Figure 2: Number of biobanks focused on a class of diseases.
Type of collected biological samples
The major aim of an organization concentrating on biobanking is the collection of human samples. Currently, the International Society for Biological and Environmental Repositories (ISBER) describes all aspects related to biobanking activity on an international level, encouraging harmonization. Moreover, biobanks in the U.S.A. operate according to the regulations of the Food and Drug Administration (FDA) and the tissue standards of the Centers for Disease Control and Prevention (CDC) and various professional associations, while the activity of similarly oriented entities in Europe is regulated by European Union (EU) recommendations [16,17]. On the other hand, procedures carried out by individual European biobanks vary notably according to protocols established by partnerships/networks of biobanks across Europe [18].
Noteworthy samples procured by biobanks are nucleic acids (DNA, RNA) and cell lines, DNA and cell lines being the most frequently exchanged according to Hirtzlin et al. [9], solid tissues (e.g., brain, muscle tissue), human waste products (e.g., urine, stool) and other biological fluids (e.g., saliva, amniotic fluid) as well as blood samples. Blood samples include anticoagulated (plasma, buffy coat), whole blood or coagulated (serum) blood [16]. The specimens are retrieved from healthy or diseased donors and are categorized in various types (anonymous, anonymized, identifiable, identified) of collections [19]. Preservation procedures might include formalin fixation or other methods, followed by freezing at 4°C, -20°C, -80°C or -190°C [10,20], while Hirtzlin et al. [9] differentiate the method regarding cells and cell lines, mentioning the use of liquid nitrogen. Formalin-fixed paraffin embedded (FFPE) samples are the easiest to store and hence the most common, despite often being considered unsuitable for modern genome research [14,21,22]. On the other hand, cryopreservation requires a specialized process and rapid coordination between medical personnel in order to prevent counter-effects in gene expression due to tissue ischemia [14,23,24] while the hypothermic storage outcome needs to continuously improve in order to meet the advancing needs in biomedical research [25]. In addition, quality control procedures are essential. The integrity of nucleic acids should be assessed using electrophoretic analysis, paraffin embedded tissue can be checked by immune histo chemical staining, while liquid nitrogen should be regularly replenished [26-28].
It should be noted that the collection process of specimen types differs extensively depending on the type of sample, the nature of the study, the physical state of the donor, the national research guidelines and the legal and ethics framework [1,29].
Various protocols dictate DNA extraction and storage. DNA from anti-coagulated venous blood, exponentially multiplied by PCR [30], followed by its cryopreservation at -80oC9 is a routine method in many laboratories. However, practices vary, for example, Psifidi et al. [31] have exemplified that retrieving DNA from buffy coat instead of whole blood demonstrates significant advantages in maintaining its quality.
RNA can be extracted from whole blood tubes, containing heparin and initially stored at -20°C [32]. Alternatives include RNA isolation from paraffin blocks or frozen tissue. However, multiple studies have shown that RNA from FFPE samples is inadequate for molecular studies, due to the chemical alterations caused by paraffin with several studies providing possible approaches to recover intact and amplifiable DNA [33-37].
Musculoskeletal tissues must be excised within 12 hours after death if the donor’s body has not been refrigerated or less than 24 hours if it has been [38]. Brain tissue samples are removed postmortem with a delay of 2-6 hours and are frozen and stored at -70°C or -80°C [39].
One of the objectives of this survey is to specify the type of material stored in the respective biobanks. Types included DNA, RNA, whole blood, serum, plasma, tissues (cryo or paraffin preserved), and cell lines. Fifty six out of the 56 biobanks provided information on their type and number of collected specimens (Table 1).
| Type of collected biological samples |
Number of biobanks |
Number of biobanks (%) |
| DNA |
38 |
68% |
| RNA |
17 |
30% |
| Blood |
13 |
23% |
| Serum |
20 |
36% |
| Plasma |
21 |
38% |
| Tissues |
37 |
66% |
| Cell lines |
25 |
45% |
Table 1: Type of material stored by 56 European biobanking organizations
Geographical location of biobanks
The first successful attempts to collect and effectively store human specimens were conducted over 100 years ago, while bio repositories encompassing these collections were significantly increased in number during the last two decades [29]. As a result, the geographical allocation of these institutions is remarkably widespread, with large-scale biobanks in the United States, Japan, Korea and several European countries. Until now, the top 6 countries according to sample sizes assembled by the corresponding biobanks are the United Kingdom, United States, Sweden, France, the Netherlands and Italy [7].
Information received from the two pan-European biobank networks, about the location of their biobank-members, are organized in Figure 3.
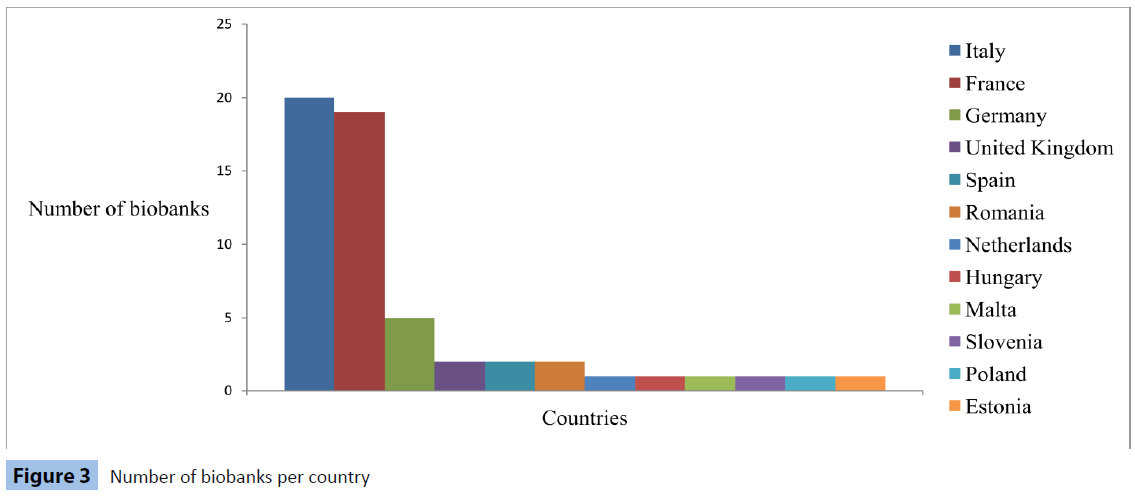
Figure 3: Number of biobanks per country
Number of Recent Publications
Scientific progress is strongly connected to the interdisciplinary cooperation of different research teams. This collaborative nature of the majority of the latest biomedical projects requires broad networking for the dissemination of relevant data. With the realization of the multifactorial genetics that many disorders present, medical research has been focused on the study of pathological features and their relation with various environmental factors [1]. In order to achieve this approach, the collection of body tissues, originally viewed as a method for macro-anatomical studies, has taken a pivotal new role. Assembling and examining these specimens is essential for the purpose of research focused on genomics or proteomics [7] and offers scholars the chance to indirectly study the effects of the environmental variables (e.g., weather, temperature, social and educational background) on a disparate sum of samples, collected by donors of various origins.
In order to evaluate the results, peer-review has been the touchstone of the scientific method since the 20th century [40]. Moreover, a biobank’s primary purpose is to support contemporary research by providing aspiring scientists with biological materials. Taking into account the above facts, we propose that a biobank’s contribution to scientific advancement is estimated by the number of scientific papers published by their distinctive associated research parties in peer-reviewed journals.
It has been stated that our main index on selecting the biobanks constituting our sample is the number of published work in which they collaborated utilizing their biological collections. The data collected from the BBMRI database are publications from a 5-year period, between 2007 and 2012. Accordingly, the ones used from Euro BioBank are under the sector “Key publications”, found in every biobank’s profile page. Proceeding in cross-examining the previous factors that characterize a biobank and the aforementioned indicator of scientific output, 2 sub-categories are created, which will be analyzed in the following paragraphs.
Research focus of biobank-Number of recent publications
The aim of this statistical comparison is to determine the fields of ongoing research and to classify them by a descending order related to the number of published work. The method followed can be summarized in the following steps:
All the disease groups (according to the ICD) were listed.
The information page of every biobank-member was examined. The research field(s) of each biobank was noted and the final results were pictorially depicted in Figure 1.
The numerous paper publications in peer-reviewed journals (regardless of impact factor), following the process mentioned in the section II. Number of recent publications, were concentrated.
A statistical approach has been used in order to approximately calculate the number of publications, centered on each disease category from the 18 available (Step 1). The utilized method is based on 2 hypotheses:
The research focus (disease group) in every biobank’s information sheet, catalogued in the 2 networks, directly indicates that the biobank’s primary scientific interest, resources, research collaborations and experimental efforts are directed towards this specific field.
We assume that in case of biobanks concerned with multiple disease groups, research activities resulting in published articles would be divided according to the exact number of the corresponding fields. However, due to the distinct pathological features of every disease as well as the different regulations of every country, the internal bylaws of every biobank and associated research teams, the various collections of specimens and the diverse analyzing methods utilized, we result in an unequal allocation of a biobank’s publications among the multiple disease groups/interests.
Contemplating the above thesis, we postulate, that even if the biobank’s publications are not apportioned precisely in every field, a number of them, will be found in each group of disorders. This is further supported by the fact that the biobanking activity of a biorepository is focused on the fields listed in its information page, as it is stated in our first hypothesis.
Taking into account these facts, we aim to statistically estimate for every biobank, the minimum/maximum portion of the total publications, which is expected to be related to the number of the disease categories constituting its research focus. In order to accomplish the above, without loss of generality, we define a minimum coefficient, which will be calculated by the following formula:
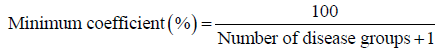
As a result, the maximum value of each coefficient can be calculated:
Maximum coefficient (%) =100 − (Number of disease groups −1)*Minimum coefficient
The corresponding coefficients are tabulated in Table 2.
| Number of disease groups |
Coefficient (Minimum-Maximum) |
| 2 |
33.3-66.7 |
| 3 |
25-50 |
| 4 |
20-40 |
| 5 |
16.7-33 |
| 6 |
14.3-29 |
| 7 |
12.5-25 |
| 8 |
11.1-22 |
| 9 |
10-20 |
| 10 |
9-19 |
| 11 |
8.3-17 |
| 12 |
7.7-15.3 |
| 13 |
7.1-14.8 |
| 14 |
6.7-12.9 |
| 15 |
6.3-11.8 |
| 16 |
5.9-11.5 |
| 17 |
5.6-10.4 |
| 18 |
5.3-9.9 |
Table 2: Minimum/maximum values of coefficients (%), according to the number of disease groups forming a biobank’s research focus.
Utilizing the above coefficients, the minimum-maximum number of publications in every disease category for every biobank has been estimated:

Finally, we calculate the average value in every disease category for every biobank and we sum them, depicting the results in Figure 4.
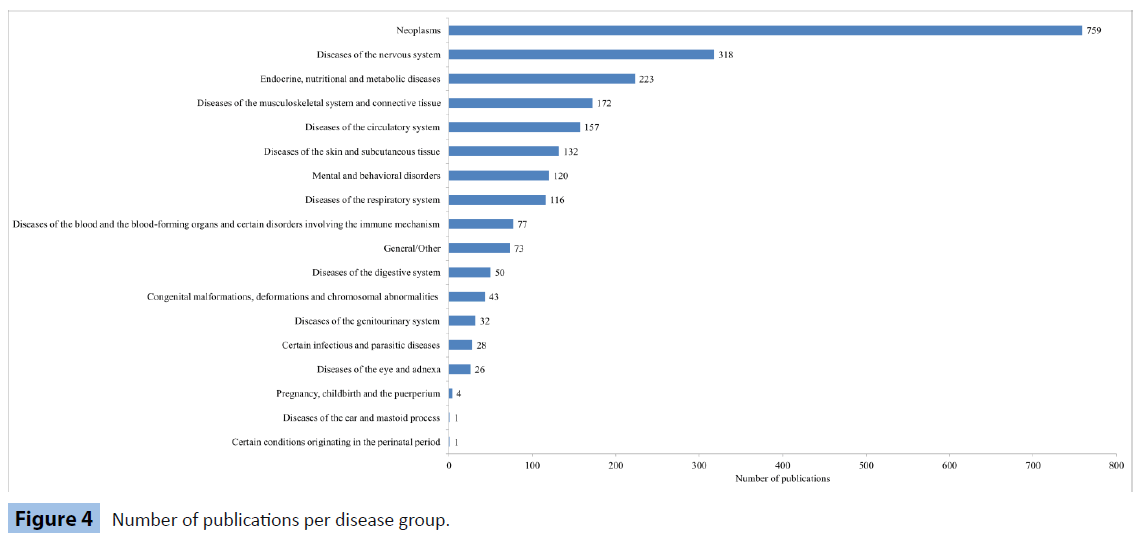
Figure 4: Number of publications per disease group.
Visually comparing Figure 2 with Figure 4, the strong similarity in the two graphs is apparent. Moreover, the following graph (Figure 5) hints a possible analogical relation between the 2 variables.
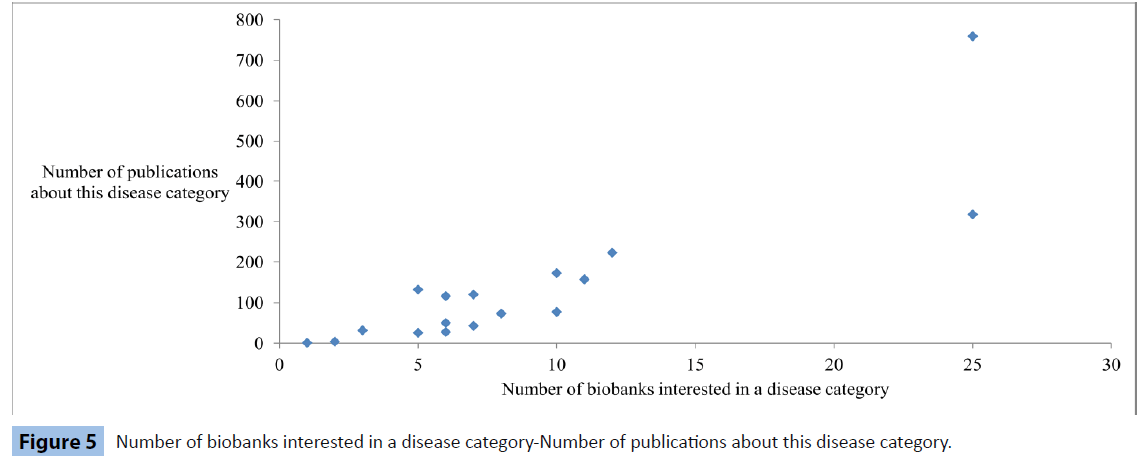
Figure 5: Number of biobanks interested in a disease category-Number of publications about this disease category.
In order to accurately examine the existence of any correlation between the 2 variables, we use Pearson product-moment correlation coefficient (Pearson rxy):
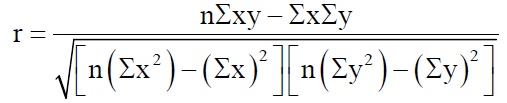
where n is the number of the value pairs, x is the number of biobanks focused on a disease group and y is the number of publications in a disease group.
In addition, Student’s t-test was used to determine significance:
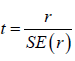
where r is the Pearson coefficient and 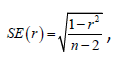 the standard deviation.
the standard deviation.
We estimate that t=7.13 (n = 18; P<10-5) which determines a statistically significant correlation between the 2 variables; number of biobanks with an interest in a disease group-number of publications related to this group.
Spherically estimating the above observations, we can hypothesize that progress in most fields of medical and biological sciences is highly dependent on the number of institutions concerned with the gathering of biomaterials and tissue samples. A higher activity of biobanking organizations in one research field is expressed with increased interdisciplinary collaborations between research associations (basic or clinical) and them. We can safely assume that this concentrated attention is going to be depicted as a higher number of published articles corresponding to unresolved issues in this field, thus directly advancing science. In addition, having statistically established a bidirectional link, we propose that biobanks, besides operating as repositories which provide research material supplements and support research teams, can be viewed as numerical representations of a medical field’s research progress. Biomedical research efforts have reached a turning point, where the existence of sufficient samples and rapid utilization methods are essential factors in order to achieve fruitful and generally implemented results. Consequently, without biobanking expertise, major difficulties arise, for example in researching multifactorial diseases, which are about to be overcome with the study of the assorted resources procured by developed biorepositories. Concluding, various questions remain obscured. Is there a need for more biobanks focused on disease groups currently receiving minimal attention or these sectors are not dependent on sample utilization? On the other hand, are distribution issues an evident issue, indicating the urgency to enhance collaborative relations between these entities? We propose the conduction of additional surveys, aiming to clearly identify the role of biobanks in optimizing and advancing modern research.
Type of collected biological samples-number of recent publications
The kinds of specimens assembled by biobanks vary, from macromolecules like DNA to whole tissue parts. By reviewing the different types of collections in a biobank’s catalogue in comparison with the organization’s scientific output, we hope to address the question; which type of specimen is most often used in research projects?
In order to provide an answer, we need to overcome 2 obstacles: First, there is a high chance of using more than one type of biomaterial in a research project. Secondly, even if a positive correlation between a specific biospecimen and the number of published articles is determined, how can we ascertain if this sample is the one solely used in these studies?
One empirical way to display the second issue is by examining Figure 6, which indicates that collectively, biobanks which store DNA, are those which contribute to the most publications, regardless of the fact that this nucleic acid may not be utilized in all these research efforts. We can interpret this graph by considering that some tissue types (DNA, tissues) seem to present more favorable extraction methods and higher frequency as samples that donors consent to provide, compared to others (blood, RNA). However, we are unable to claim the existence of a correlation between the number of collected DNA samples and the scientific output of an individual biobank.
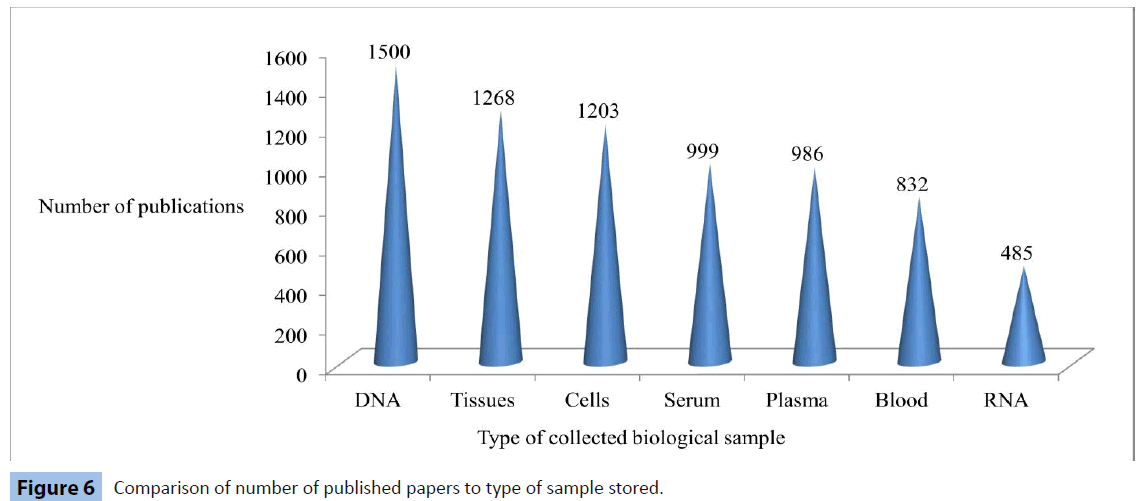
Figure 6: Comparison of number of published papers to type of sample stored.
In order to approach this matter, simultaneously with the first issue, we will follow the same statistical method used in our previous section, examining the relation between the scientific contribution of a biobank and the number of samples collected, accordingly with every type of sample (DNA, RNA, tissues, cells, blood, plasma, and serum).
Our results indicate a positive correlation between:
Number of DNA samples-Number of publications, estimated t= 2.69 (n=37; P<10-5)
Number of RNA samples-Number of publications, estimated t=5.6 (n=16; P<10-4)
Our first observations are that only DNA and RNA sample numbers are related to the amount of published research work and that RNA correlation is higher (Figures 7 and 8). On the other hand, we interpret the non-linear relation between other types of samples and the number of research papers as an indication of the multidisciplinary approach followed in biological research, where data are retrieved, under different circumstances, from several types of specimens, ranging from soft and liquid tissues to enzymes and nucleic acids. We evaluate these results as evidence that RNA collection initiatives lead to effective usage of this biomolecule to a wide spectrum of studies in several disease groups. Our analysis shows that only macromolecules, like RNA and DNA are directly linked to increased research contribution by biobanks (Figures 7 and 8), which can be alternatively expressed as a strong possibility that DNA and RNA will be frequently used as research biomaterials.
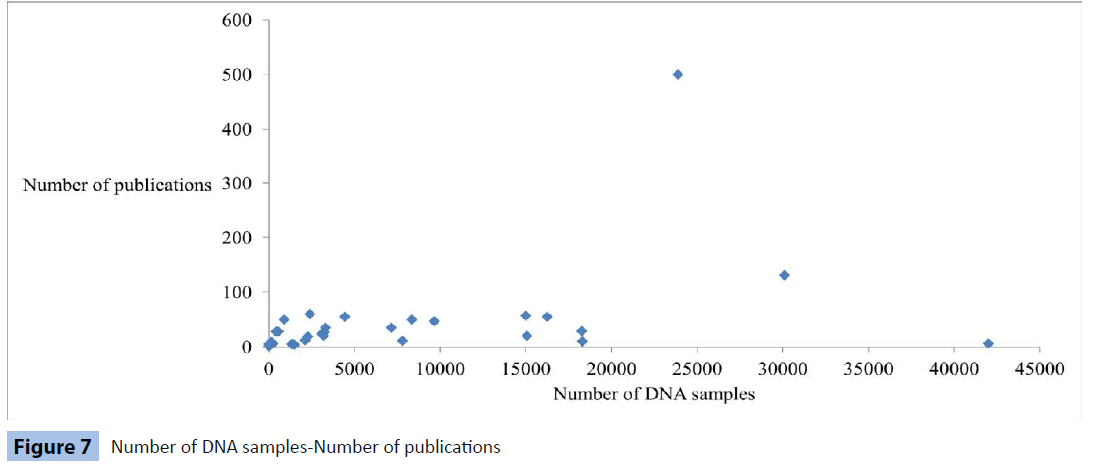
Figure 7: Number of DNA samples-Number of publications
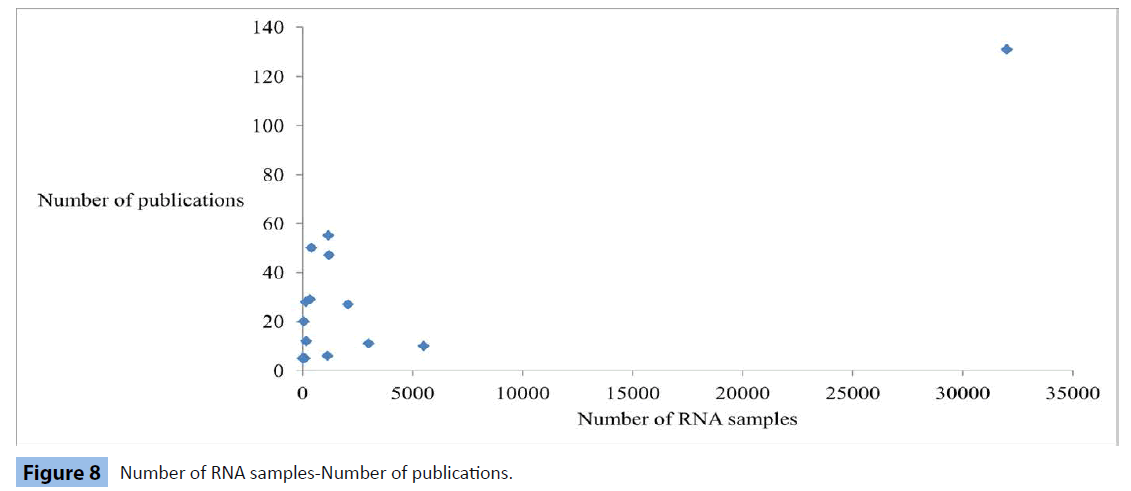
Figure 8: Number of RNA samples-Number of publications.
Discussion
Biobanking activity has been expanding across Europe, as the intersection where biotechnology methods and biomedical research needs meet. This growing interest in establishing organizations whose primary focus is the collection, categorization and distribution of human biological samples, has led to the emersion of multiple projects, seeking to harmonize and unite biobanks under a common scientific and legal framework.
In this paper, we described both key aspects of a biobank’s operations, as well as a statistical way of interpreting the ambiguous relation between these aspects/characteristics of a biobank and its purpose/objective, scientific progress.
We propose the initiation of a joint effort from all active European biobanks in order to create a set of criteria for biobank characterization. It is expected that, as biobanking organizing attempts are at early but evolving stages of development, complex structures, collaborations and interrelated practices will emerge [41]. Furthermore, modern challenges include the re-appraisal of many aspects, concerning ethical boundaries and legal framework [42-44]. In our review, we considered noteworthy the following criteria: type, research focus, collected biological samples and location. Encouraging the supplementation of these in future studies, we perceive the usage of specific criteria as benchmarks, as an efficient start in the way to resolve the challenges in biobank governance and stability.
Our systematic analysis has yielded results that strongly indicate the considerable contribution of biobanks to scientific efforts. In addition, the clarification of the research contribution of every sample type provided by European biobanks to facilitated institutions is the first step to harmonize the scientific interchange between them. Appraising the characteristics of the precise role of biobanks in research activities is a precondition in order to render these organizations effective assets in biomedical progress worldwide.
Acknowledgements
This study was conducted under the guidance and advisory support of the Panhellenic Biobank of Neurological Disorders.
9575
References
- Zika E, Paci D, Schulte in den Bäumen T, Anette Braun, Sylvie RijKers-Defrasne, et al. (2015) Biobanks in Europe: Prospects for harmonisation and networking JRC The institute for prospective technological studies 2010.
- Hawkins AK (2010) Biobanks: importance, implications and opportunities for genetic counselors. J Genet Couns 19: 423-429.
- Ginsburg GS, Burke TW, Febbo P (2008) Centralized Biorepositories for Genetic and Genomic Research. AMA 299: 1359-1361.
- Henderson GE, Cadigan JR, Edwards TP, Conlon I, Nelson AG, et al. (2013) Characterizing biobank organizations in the U.S.: results from a national survey. Genome Medicine 5: 3.
- Kang B, Park J, Cho S, Lee M, Kim N, et al. (2013) Current status, challenges, policies, and bioethics of biobanks. Genomics Inform 11: 211–217.
- Yuille M, Jan van Ommen G, Brèchot C, Cambon-Thomsen A, Dagher G, et al. (2008) Biobanking for Europe. Brief Bioinform 9: 14-24.
- Hirtzlin I, Dubreuil C, Prèaubert N (2003) An empirical survey on biobanking of human genetic material and data in six EU countries. European Journal of Human Genetics 11: 475–488.
- Watson PH, Barnes RO (2011) A proposed schema for classifying human research biobanks. Biopreservation and Biobanking 9: 327-333.
- Lenk C, Sándor J, Gordijn B (2011) Biobanks and tissue research: The public, the patient and the regulation Springer Science & Business Media.
- Riegman PH, Dinjens WN, Oosterhuis JW (2007) Biobanking for interdisciplinary clinical research. Athobiology 74: 239–244.
- Swede H, Stone CL, Norwood AR (2007) National population-based biobanks for genetic research Genet Med 9: 141–149.
- Asslaber M, Zatloukal K (2007) Biobanks: transnational, European and global networks. Brief Funct Genomic Proteomic 6: 193-201.
- World Health Organization International Statistical Classification of Diseases and Related Health Problems 10th Revision.
- Campbell LD (2011) Best practices for repositories: collection, storage, retrieval and distribution of biological materials for research International Society for Biological and Environmental Repositories (ISBER).
- Council of Europe Committee on Bioethics Working document on research on biological materials of human origin. Council of Europe 2014.
- Wichmann E (2010) Need for guidelines for standardized. biobanking Biopreservation and Biobanking 8: 1.
- European Society of Human Genetics Data storage and DNA banking for biomedical research: technical, social and ethical issues European Journal of Human Genetics 11:8–10.
- Mager SR, Oomen MH, Morente MM, Ratcliffe C, Knox K, et al. (2007) Standard operating procedure for the collection of fresh frozen tissue samples. Eur J Cancer 43: 828-834.
- Ericsson C, Franzén B, Nistér M (2006) Frozen tissue biobanks. Tissue handling, cryopreservation, extraction, and use for proteomic analysis. ActaOncol 45: 643-661.
- Dash A, Maine IP, Varambally S, Shen R, Chinnaiyan AM, et al. (2002) Changes in differential gene expression because of warm ischemia time of radical prostatectomy specimens. Am J Pathol 161: 1743–1748.
- Spruessel A, Steimann G, Jung M, Lee SA, Carr T, et al (2004) Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques 36: 1030-1037.
- Baust JG, Gao D, Baust JM (2009) Cryopreservation: An emerging paradigm change. Organogenesis 5: 90-96.
- Battifora H (1991) Assessment of antigen damage in immunohistochemistry. The vimentin internal control. American Journal of Clinical Pathology 96: 669-671.
- Bricarelli FD, Baldo C, Filocamo M (2015) Guidelines for genetic biobanks. Telethon network of genetic biobanks 2004.
- Guerin JS, Murray DW, McGrath MM, Yuille MA, McPartlin JM, et al. (2010) Molecular medicine Ireland guidelines for standardized biobanking Biopreservation and Biobanking;8: 3-63
- Eiseman E, Haga SB (1999) Handbook of Human Tissue Sources. A National Resource of Human Tissue Samples. RAND Corporation .
- Yates JR, Malcolm S, Read AP (1989) Guidelines for DNA banking. Report of the clinical genetics society working party on DNA banking J Med Genet 26: 245-250.
- Psifidi A, Dovas CI, Bramis G, Lazou T, Russel CL, et al. (2015) Comparison of Eleven Methods for Genomic DNA Extraction Suitable for Large-Scale Whole-Genome Genotyping and Long-Term DNA Banking Using Blood Samples. PLoS ONE 10: e0115960.
- Willemsen G, De Geus EJ, Bartels M, van Beijsterveldt CE, Brooks AI, et al. (2010) The Netherlands twin register biobank: A resource for genetic epidemiological studies. Twin Research and Human Genetics 13: 231-245.
- Stanta G, Schneider C (1991) RNA extracted from paraffin embedded human tissues is amenable to analysis by PCR amplification. Biotechniques 11: 304-308.
- Krafft AE, Duncan BW, Bijwaard KE, Taubenberger JK, Lichy JH (1997) Optimization of the Isolation and Amplification of RNA From Formalin-fixed, Paraffin-embedded Tissue: TheArmed Forces Institute of Pathology Experience and Literature Review. Mol Diagn 2:217-230
- Goldsworthy SM, Stockton PS, Trempus CS, Foley JF, Maronpot RR (1999) Effects of fixation on RNA extraction and amplification from laser capture micro dissected tissue. Mol Carcinog 25: 86-89.
- Masuda N, Ohnishi T, Kawamoto S, Monden M, Okubo K (1999) Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res 27: 4436-4443.
- Rait VK, Zhang Q, Fabris D, Mason JT, O'Leary TJ (2006) Conversions of formaldehyde-modified 2'-deoxyadenosine 5'-monophosphate in conditions modeling formalin-fixed tissue dehydration. J Histochem Cytochem 54: 301-310.
- Malinin Theodore I (2008) Principles of Musculoskeletal Tissue Banking In: Pietrzak WS Musculoskeletal tissue regeneration: Biological materials and method. Humana Press, New York 483-508.
- Spier R (2002) The history of the peer-review process. Trends in biotechnology 20: 357-358.
- Aldrich HE, Ruef M (2006) Organizations evolving. SAGE Publications, London.
- Cambon-Thomsen A, Rial-Sebbag E, Knoppers BM (2007) Trends in ethical and legal frameworks for the use of human biobanks. EurRespir J 30: 373–382.
- Greely HT (2007) The uneasy ethical and legal underpinnings of large-scale genomic biobanks. Annu. Rev. Genomics Hum Genet 8: 343–364.
- Hansson MG (2009) Ethics and biobanks. British Journal of Cancer 100: 8–12.










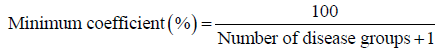



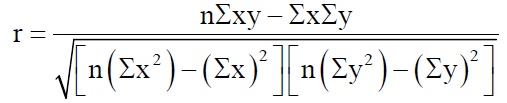
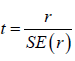
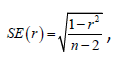 the standard deviation.
the standard deviation.

