Introduction
Individuals who suffer from atrial fibrillation are at significantly increased risk of morbidity and mortality, especially from complications of thromboemboli, including strokes, as a result of independent risk factors inherent to atrial fibrillation [1]. This risk is amplified when individuals experience atrial fibrillation in conjunction with other pre-existing conditions such as smoking, hypertension, previous myocardial infractions, valvular heart diseases, congestive cardiac failure, diabetes, and other conditions. As a result, therapy aimed at returning the arrhythmic depolarization of the atria to a normal rhythm is necessary.
In 1780, an Italian physician named Luigi Galvani made one of the first discoveries of the relationship between muscular contraction and electricity when he noticed the legs twitching of a dead frog upon the application of electric charge [2]. Twelve years later, the British scientist, James Curry, published a paper describing the potential to successfully “rouse the heart to act [3], “essentially inventing the first cardiac defibrillator. Further research was conducted over the centuries culminating in the alternating current defibrillator developed by Paul Zoll of Beth Israel Hospital and Harvard Medical School in 1956, on which modern devices are based. And three years after Zoll’s development, in February 1959, two Russian physician-scientists, developed their own apparatus and published the first known case of direct current cardioversion for atrial fibrillation [4]. Since then, direct current (DC) cardioversion has been extensively used as a treatment for returning the atria to a normal rhythm.
In patients with atrial fibrillation, it is hypothesized that the atria often experience a re-entrant circuit which causes the heart muscle to continually become depolarised in rapid succession. Sometimes there may even be multiple re-entrant loops which generate an even more rapid and chaotic rhythm of depolarisations resulting in atrial fibrillation [5]. DC cardioversion works by generating a transitory depolarization of nearly all cardiac myocytes within the heart. This results in termination of any errant re-entrant loops which essentially allows the heart to reset to a normal sinus rhythm [6].
Direct current cardioversion is by far the most successful and safest therapy for returning the rhythm of patients in atrial fibrillation to a normal sinus rhythm [7]. It is for this reason it is vital for all physicians to understand its use and success as therapy for prevention of strokes and other complications of atrial fibrillation, and to maximise patient access to this crucial therapy.
Rationale and Objectives
Audits regarding the access to and the success of DC cardioversion have not been published to date and there is minimal data available regarding the success of DC cardioversion within the region. This audit was undertaken to evaluate the success of DC cardioversion for the prevention of complications related to atrial fibrillation for the five-year period between 2009 and 2013 within the Barking, Havering, and Redbridge University Hospitals Trust (BHRUT).
Audit Standards
Waiting times was the first standard that we wished to evaluate. Due to the variability of patients, hospital resources, access to healthcare, and patient care standards within different areas, we chose to set the standard for waiting time to anti-coagulation at one week. The success rate at the time of cardioversion found during literature review spanned from 54% to 87% [8-10]. Therefore, our success rate standard was set to surpass this range at 87%.
To determine success of six-weeks post cardioversion, we found there were minimal studies citing a follow-up at this time period. For this reason, we considered a range of four to eight-weeks for follow-up to determine success. We found in the literature that this ranged from 37% to 51% [8,11-13]. We therefore set this standard at greater than 51%. Finally, literature search was conducted to determine the incidence of complications in DC cardioversion. The proportion of cases of DC cardioversion conducted which resulted in complications was found to range from 0% to 0.9% [14-16]. For this reason, a standard of less than 1% of cases resulting in complications was selected.
Materials and Methods
DC cardioversion is performed at Queen’s Hospital, the primary hospital within BHRUT, for all patients referred to the Trust. It is performed as an elective procedure for patients and has been led by specialty nurses since 2008 in accordance with regional guidelines. A retrospective review of all DC cardioversions that were performed between 2009 and 2013 within BHRUT was completed. The data was obtained through the Queen’s Hospital Data Centre which is responsible for procuring records for all clinical audits conducted within the Trust. A total of 729 patients were referred for DC cardioversion in the aforementioned five-year period. Of these 729 patients, 531 patients received cardioversion. The remaining patients either declined the procedure, or were unacceptable for DC cardioversion due to declining to undergo anticoagulation treatment, were already in sinus rhythm, received ablation therapy, had an LAA thrombus, or had the procedure done elsewhere. Anti-coagulation was carried out by means of warfarin, dabigatran, or low molecular weight heparin (enoxaparin). Of the patients anti-coagulated, 511 patients were anti-coagulated via warfarin, 3 patients were anti-coagulated with dabigatran, and the remaining 17 patients were anti-coagulated with LMWH.
Results
Patients selected for cardioversion were between the ages of 20 and 86 with a mean age of 62.6. Figure 1 shows histogram of patient age groups of individuals who were cardioverted. Of the patients who were cardioverted, 399 patients were male and 132 patients were female. The ethnic breakdown was also monitored and recorded. The proportion of Caucasians was within 6% of the population of the largest London borough from which patients originated [17]. As expected based on this population data, the majority of the patients were Caucasian (494 patients). The remainder of the patients were divided into Asian (21 patients), Afro Caribbean (4 patients), East Asian (3 patients), and other (9 patients). Excluding Caucasians, the vast majority of cardioverted patients were of Asian descent (56.3% of non-Caucasian patients).
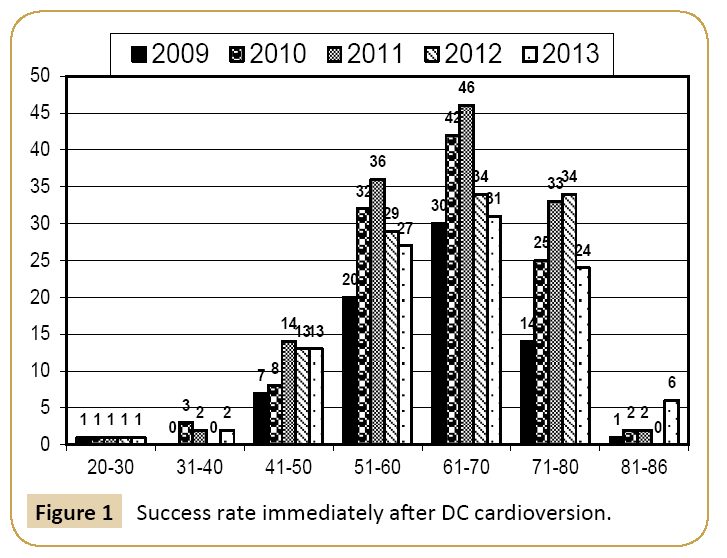
Figure 1: Success rate immediately after DC cardioversion.
Waiting times for treatment with DC cardioversion were also tracked. Waiting time begins at the time a patient is referred by a cardiologist to the time anti-coagulation is initiated. Far exceeding regional standards, most patients were cardioverted with a waiting time of less than one week for all five years of this audit [18]. Furthermore, those patients waiting for greater than three weeks include instances in which a patients request to delay starting as well as requests returned to the cardiologists for reasons other than patient backlog. This included instances in which delays were caused by improperly completed paperwork. Moreover, the time between reaching therapeutic anticoagulation to the time cardioversion therapy was delivered was between five to ten days for all five years of the audit. Therapeutic anti-coagulation is defined as the point at which patients reach an international normalized ratio of 2-3 sustained for a period of at least four weeks. The waiting times are summarised and broken down by year in Figure 2. The DC cardioversion success rate can be divided into that immediately after the delivery of therapy and at six-weeks follow-up. For the five-year period, the average success rates each year of DC cardioversion among patients ranged from 84% in 2009 to 94% in 2010. The success rates by year are summarised in Figure 3.
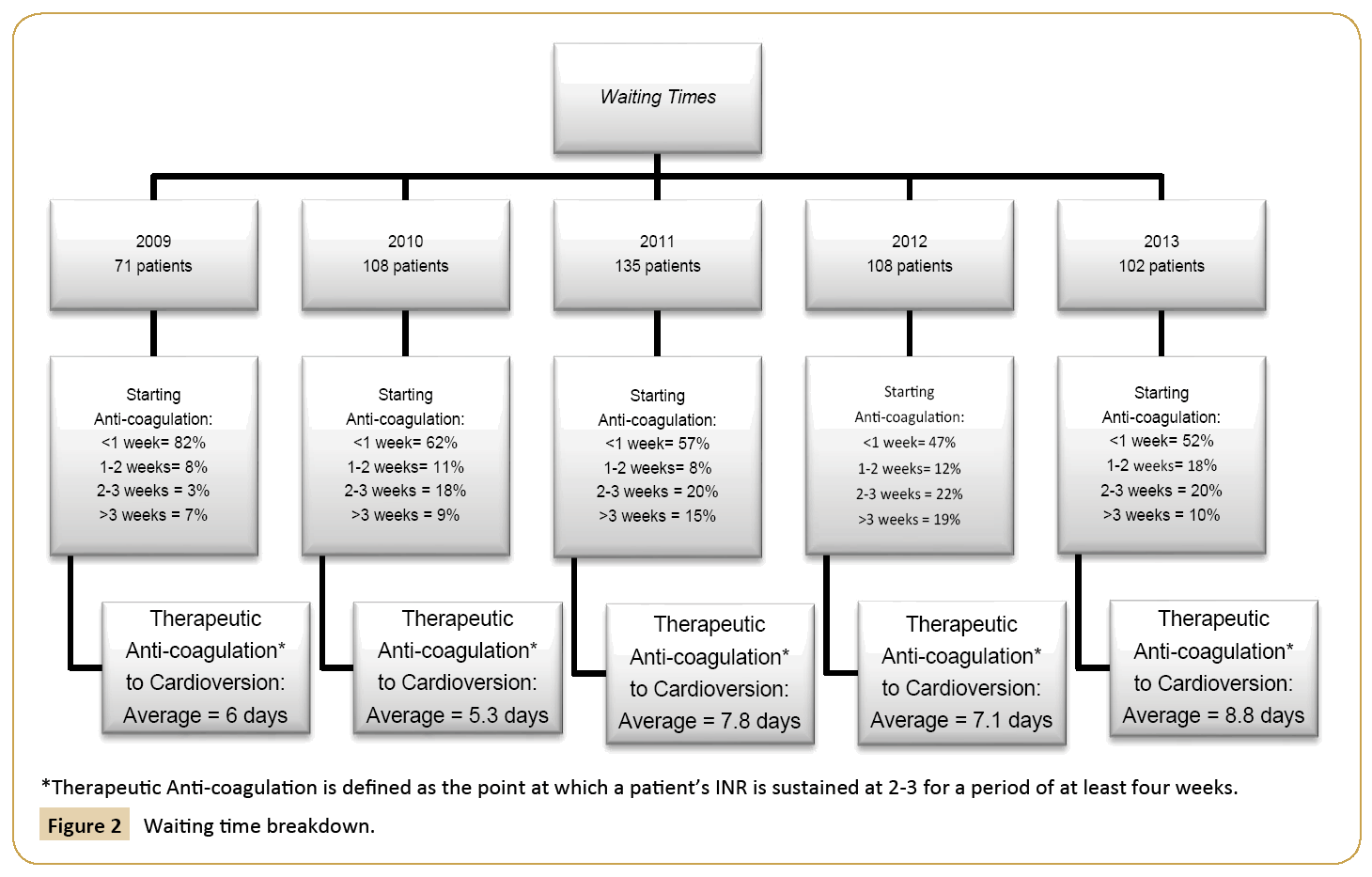
Figure 2: Waiting time breakdown.
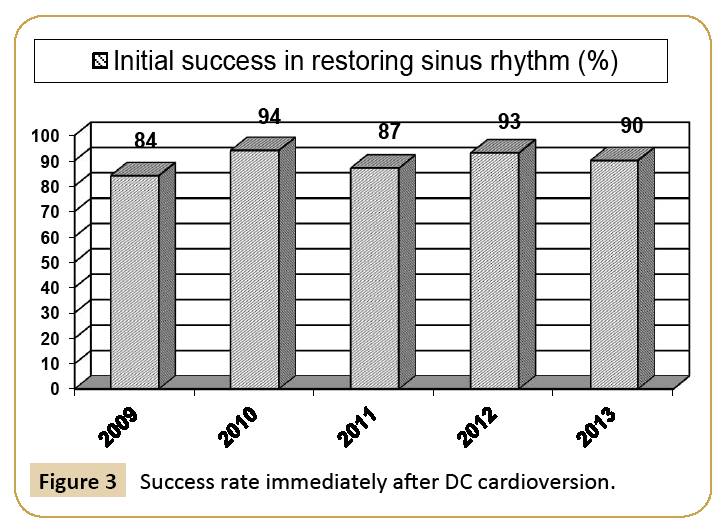
Figure 3: Success rate immediately after DC cardioversion.
Furthermore, we were able to follow-up with our patients at six weeks to observe continued success at this time. Our success rates ranged from 59% to 61% at six-weeks follow-up. The success rate at follow-up may be viewed in Figure 4. For the five years of the audit, there were zero complications as a result of DC cardioversion delivered within the Trust.
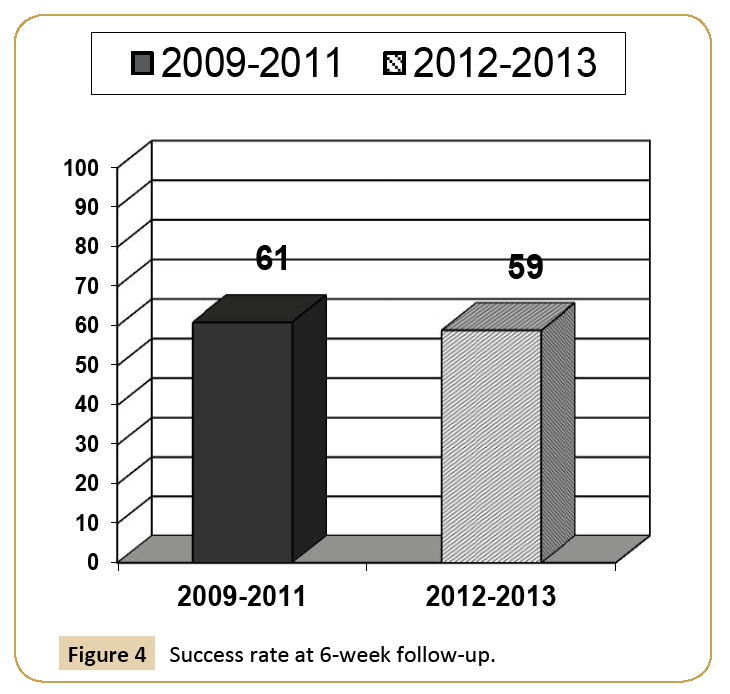
Figure 4: Success rate at 6-week follow-up.
Consequently, there were no hospital admissions or over-night stays required as a result of patients undergoing DC cardioversion within the Trust.
Discussion
Due to the haemostasis which occurs within the atria of patients with atrial fibrillation, it is very common for these patients to suffer from mural thrombi generating thrombotic emboli which may travel to the brain or lungs thereby increasing the risk of strokes thromboembolic events including strokes [19]. The risk for such an event may be estimated by means of a point system referred to as the CHA2DS2-VASc score [20]. CHA2DS2-VASc is an acronym which identifies seven risk factors: namely, presence of Congestive cardiac failure/left ventricular dysfunction (ejection fraction <35%), hypertension, age, presence of diabetes mellitus, previous history of strokes, TIAs, or thromboemboli, female sex, and presence of vascular disease such as prior MIs, peripheral artery disease, or aortic plaques. The risk is then stratified into three levels: low, intermediate, and high risk based on the sum of the points identified by the score. A score of zero is considered low risk, a score of one is considered intermediate risk, and a score of more than one is considered high risk [20,21]. Though some of the risk factors in the CHA2DS2-VASc score are modifiable, the majority are not. Therefore, the success of therapy such as DC cardioversion is necessary as secondary prevention for strokes and other thromboembolic events.
Overall, the percentage of patients in this audit who were converted to sinus rhythm and the patients who remained in sinus rhythm after six weeks was extremely impressive. The success rate immediately after delivery of therapy was very high at 84% to 94% which was primarily at or above the range found within our literature review (54% to 87%) [8-10]. Furthermore, the success rate was sustained for 59% to 61% of patients at sixweeks follow-up. This too was extremely impressive considering that the rates found during our literature review at similar time scales was 37% to 51% [8,11-13].
There are a number of factors which are considered by cardiologists to maximise the possibility of successful DC cardioversion. One such factor frequently cited by cardiologists is the amount of left atrial dilatation present in the patient. Szulc and Torbicki [9] found in their prospective study involving 150 consecutive patients undergoing elective DC cardioversion that while patients who spontaneously converted back to sinus rhythm did tend to have smaller atria, patient who fail to respond to DC cardioversion did not have a statistically significant increase in atrium size compared to those who responded to DC cardioversion [22]. One factor which does play a significant role in determining success of DC cardioversion is whether the patient has previously had any failed attempts at being converted back to sinus rhythm. The chance of success after a failed attempt drops off sharply as the number of attempts increases with the benefit of DC cardioversion plummeting to a statistically insignificant effect by the fourth attempt [23]. Duration of atrial fibrillation is another important factor that influences the outcome of DC cardioversion. It has been shown that patients who have been in persistent atrial fibrillation for a long duration (>6 months) have a substantially reduced success rate for being converted back to sinus rhythm, and that a short duration of persistent atrial fibrillation (<6 months) shows a statistically significant increase in the success rate for conversion back to sinus rhythm [12].
Obesity is one of the fastest growing epidemics in the western world. Unfortunately, people who are obese tend to have other medical problems as well, such as cardiac arrhythmias. As such, obesity is another factor which is important to consider for patients requiring DC cardioversion. Success of DC cardioversion has regrettably been shown to be reduced in obese individuals [12]. Obese patients generally have larger amounts of insulating adipose tissue beneath their skin. It has therefore been hypothesized that failure of DC cardioversion in obese patients may be due, at least in part, to increased tissue through which an electric current must traverse and increased surface area [24].
Patients with atrial fibrillation secondary to valvular heart disease are at a significantly increased risk for thrombotic emboli compared to those with atrial fibrillation with other aetiologies. However, DC cardioversion is frequently unsuccessful in patients with valvular atrial fibrillation [25]. Instead repair of the valvular defect by valvuloplasty and posterior atrial wall ablation, and other surgical consideration yield better long term maintenance of sinus rhythm in these patients if they are candidates for such procedures [26]. It is thought that the pathophysiology behind this type of atrial fibrillation is due to atrial dilatation resulting from valvular problems such as mitral insufficiency, overloading the atria and thus producing increased surface area from which re-entrant loops may be generated [27]. Similarly, patients with heart failure and dilated cardiomyopathy will frequently possess dilated atria resulting in the same tendency for re-entrant loops [27]. These patients therefore suffer from similar difficulties in maintenance of sinus rhythm with treatment for atrial fibrillation as those with aforementioned valvular disease [28,29]. Therefore, when patients are referred for treatment of atrial fibrillation, careful consideration is made by the cardiologist to determine whether DC cardioversion is appropriate. It is likely that the remarkable success rates achieved within the Trust immediately after cardioversion and at six-weeks follow-up may be a direct result of the careful selection of patients based on these key factors by Trust cardiologists. Patients who do not meet selection criteria are treated with alternative options such as antiarrhythmic pharmacotherapy and atrial ablation therapy.
The primary complication of DC cardioversion is the development of a post-cardioversion thrombus [30,31]. This is particularly true in patients with an INR of less than 2.5 [31]. Because it takes time for a thrombus to form within the atria, historically for patients with acute onset atrial fibrillation, 48 h has been the limit at which they may be cardioverted without anti-coagulation. However, it has been recently shown that there is a statistically significant increased risk of a thromboembolic complication in patients cardioverted between 12 h and 24 h post onset (p=0.001, odds ratio 4.0) as compared to those cardioverted at less than 12 h post-acute onset atrial fibrillation [32]. It has further been suggested by Fatkin et al. [33] that atrial thrombi may occur as a direct result of DC cardioversion due to “atrial stunning” which may arise at the time cardioversion takes place leading to haemostatis and development of a thromboembolis [33]. Therefore, anti-coagulation is useful in prevention of thromboembolic complications related to DC cardioversion even in patients with acute onset atrial fibrillation and no evidence of a pre-cardioversion mural thrombus. When the patient is anticoagulated with warfarin, an INR ≥ 2.5 should be achieved to effectively minimise the possibility of a thrombus forming, and with the INR sustained at this level for four weeks, complications related to pre-formed thrombi should be successfully mitigated [31].
Another major complication involved in DC cardioversion is the instigation of cardiac dysrhythmias. This has been investigated extensively in the 2013 FinCV study of 7660 cardioversions conducted by Grönberg et al. [34]. This study found that the majority of dysrhythmias discovered were the result of sinus node dysfunction. The most common dysrhythmias were bradycardia and asystole (>5 second duration post cardioversion). This study also showed that the incidence of these two dysrhythmias after cardioversion where significantly higher in patients older than 65 years of age and of female sex compared to younger patients (odds ratio 1.1, p<0.0001) and males respectively (odds ratio 2.5, p=0.004). Further, patients with a history of unsuccessful cardioversions also had a statistically significant increased rate of asystole or bradycardia compared to those who have not had failed attempts (odds ratio 2.2, p=0.03). Nearly 45% of patients in the study with cardioversion induced bradyarrhythmias required the implantation of a permanent pacemaker with the main reason being sinus nodal dysfunction. It is hypothesised that the complication of post cardioversion bradycardia or asystole is rather an un-masking of sinus nodal dysfunction already present in the heart. Moreover, it is speculated that the bradyarrhythmia and atrial fibrillation present in these patients share a common origin: namely, fibrosis present in the atrial walls. This explanation is logical since patient age being positively correlated to the amount of fibrosis present in the atria (r=0.45; P<0.01), older patients would thus be at a greater risk of sinus nodal dysfunction, and would therefore result in increased instances of bradyarrhythmias [35]. The difference in risk for bradyarrhythmic complications between males and females is not understood, however females are also at an increased risk of bradycardic complication after administration of pharmacologic rhythm control [36]. In addition, it has been shown in animal studies that hypocretin-1 (HCRT1), a hypothalamic neurotransmitter in the nucleus tractus solitaries responsible for a number of autonomic functions including heart rate, is sensitive to oestrogen and that HCRT1 exposure to oestrogen results in a statistically significant reduction in heart rate compared to lack of exposure [37]. This data suggests that the effect of higher levels of oestrogen in females may be responsible for this increased risk of bradyarrhythmic complications in female patients undergoing DC cardioversion compared to males.
Pulmonary oedema has been reported as a very rare complication of DC cardioversion. This complication has been estimated at 3%, however nearly all patients with reported post cardioversion pulmonary oedema have been described as having some type of valvular defect, primarily involving mitral stenosis, or other preexisting cardiac anomalies: namely, cardiomyopathy or ischemic heart disease [38]. The mechanism behind this complication is poorly understood, but since this complication occurs after successful conversion to sinus rhythm, it has been proposed that the conversion to sinus rhythm itself may be the cause of the pulmonary oedema, particularly with restoration of sinus rhythm in the right atrium while systole remains absent in the left atrium, thereby providing for accumulation of blood within the pulmonary vasculature leading to pulmonary oedema [39].
Skin irritation or burns resulting from poor conduction of electricity between the skin and defibrillator pads or paddles is another complication which may occur during DC cardioversion. This complication is mitigated by ensuring proper use of conductive gel and proper contact of defibrillation pads to the skin minimises the occurrence of burns and irritation related to the electrical shock. Other complications include reactions to the anaesthetics used for sedation of patients or over sedation resulting in loss of respiratory drive.
Because of the continued risk of thromboembolus, it is important to minimise the waiting time for anti-coagulation. It is for this reason that we have closely monitored the time spent waiting for anti-coagulation therapy. Though we are reassured that the vast majority of patients waited for less than one week, we do find it troubling that for the period between 2009 and 2012, the proportion of patients waiting less than one week has decreased significantly each year. Furthermore, we found it more troubling that we saw that the proportion of patients waiting for anticoagulation greater than three weeks increase significantly from 2009 to 2012 only to return in 2013 to near the 2009 value. It is therefore necessary to follow-up such instances in the future to minimise their occurrences.
Comparison to National Standards
Within the United Kingdom, clinical practice is governed by the National Health Service (NHS) which sets a maximum of 18 weeks from the time a referral is made, to the time treatment is initiated for non-urgent cases [18]. Healthcare is further guided by the National Institute for Health and Care Excellence (NICE). NICE sets guidelines which are updated frequently and cover a broad range of topics and protocols. Topics covered within NICE guidelines include that of atrial fibrillation management. Accordingly, it is recommended that patients with atrial fibrillation not successfully treated pharmacologically with rate control receive prompt referral within four weeks for specialised management, including initiating anti-coagulation therapy and undergoing DC cardioversion [40]. Since anti-coagulation therapy is initiated within one week for the majority of our patients, and there are few patients whose wait time exceeds three weeks, it is clear that the Trust not only meets but exceeds these far stricter guidelines for initiating therapy. The introduction of novel oral anticoagulants (NOAC) for use in patients with atrial fibrillation undergoing DC cardioversion further reduces the waiting time to initiate anticoagulation, and also reduces the time to achieve target anticoagulation level before DC cardioversion [41].
Conclusions
The results observed in this audit were extremely encouraging with impressive success rates and complete lack of patient complications over a five-year period. We did note that some patients waited for longer than the standards we set for our audit to initiate anti-coagulation therapy, but after looking into these occurrences, we found that many of these patients chose to wait for this amount of time due to personal reasons such an upcoming holidays and the like. Though we did also identify delays generated internally within the Trust, these delays violated only our audit standards. In comparison to national requirements and regional standards, our wait times were impressively short indicating our patients spent a significantly shorter amount of time at risk for thromboembolic events than would patients waiting for times set by the NHS or NICE. To further reduce wait times in order to improve patient care beyond our already reduced rates, and further reduce risk to patients, we have initiated the use of NOACs in such patients [42]. An additional audit is required to determine a strategy to further minimise wait times longer than one week in the future and also the time to achieve therapeutic anti coagulation level before cardioversion in the patients who were on NOACs. Such strategy might also include the implementation of electronic patient records/ referrals to eliminate mishandling of referral documents, and setting up a priority queue system for atrial fibrillation patients to receive sooner anti-coagulation appointments.
Key Points
• Waiting time to initiate anti coagulation was lower and the success rate for cardioversion was higher in our audit, which were impressive compared with published literatures and local guidelines. Even this minor delay in the initiation of anti-coagulation is probably due to patients’ personal issues in addition to the paper referral for such services.
• There were no complications observed in our patients following cardioversion.
• The careful selection for patients for cardioversion with administration by highly skilled professionals enhances the success rate of cardioversion and also, limits the complications of therapy – as observed in our audit.
Future Research
• The use of NOAC will reduce the waiting time for initiation and also will reduce the time to achieve the target therapeutic anti coagulation level. Also in NOAC patients, the cardioversion can be carried out after the fixed time period after the initiation.
• The use of anti-arrhythmic drugs/pharmacotherapy may revert the atrial fibrillation to sinus rhythm.
• Future research/audit is required to find out the following:
o The waiting time for initiation of NOAC.
o The waiting time for cardioversion in NOAC patients.
o The complications after cardioversion in NOAC patients.
o The success of chemical cardioversion by correlating the use of pharmacotherapy in such patients.
Strengths and Limitations
This audit was comprised of a large number of individuals (531), which therefore generated a significant amount of data from which to analyse. Further, this audit was expansive, ranging over a period of five years thus providing for sound protection against variances which may occur throughout short-term time scales.
Though the sample size of patients was quite large, this audit was confined to a single Trust and as such, the patients originated from a single geographical region. Despite Havering (a primary catchment area for the Trust) being one of the largest boroughs in London, it is also one of the least ethnically diverse [17]. This resulted in less than eight percent of patients being of an ethnicity other than Caucasian. Due to this being a retrospective audit, we were unfortunately unable to extract data for the majority of our patients regarding the use of antiarrhythmic medications prior to the delivery of DC cardioversion therapy. Therefore, we were unable to correlate the use of such pharmacotherapy with success or failure to convert back to sinus rhythm.
14765
References
- Benjamin EJ, Levy D, D’Agostino RB, Silbershatz H, Kannel W, et al. (1998) Impact of Atrial Fibrillation on the Risk of Death. Circulation 98: 946-952.
- Marco B (1998) Medicine and science in the life of Luigi Galvani. Brain Res Bull 46: 367-380.
- Cakulev I, Efimov IR, Waldo AL (2009) Cardioversion: Past, Present, and Future. Circulation 120: 1623-1632.
- Vishnevskii AA, Tsukerman BM, Smelovskii SI (1959) Control of fibrillating arrhythmia by the method of electrical defibrillation of the atrium. Klin Med (Mosk) 37: 26-29.
- Veenhuyzen GD, Simpson CS, Abdollah H (2005) Inhibition of the Renin-Angiotensin System and Atrial Fibrillation in Heart Failure. Heart Drug 171: 755-760.
- Lown B (2002) Defibrillation and cardioversion. Cardiovascres 55: 220-224.
- Gorenek B (2012) Cardioversion in Atrial Fibrillation Described. E J Cardiol Pract 11.
- Sandler DA (2010) Whatever happens to the cardioverted? An audit of the success of direct current cardioversion for atrial fibrillation in a district general hospital over a period of four years. Br J Cardiol 17: 86-88.
- Szulc M, Torbicki AA (2001) Factors related to the early and late success of direct current cardioversion of chronic nonrheumatic atrial fibrillation: An echocardiographic study. Exp Clin Cardiol 6: 200-205.
- Botto GL, Politi A, Bonini W, Broffoni T, Bonatti R (1999) External cardioversion of atrial fibrillation: role of paddle position on technical efficacy and energy requirements. Heart 82: 726-730.
- Channer KS, Birchall A, Steeds RP, Walters SJ, Yeo WW, et al. (2003) A randomized placebo-controlled trial of pre-treatment and short- or long-term maintenance therapy with amiodarone supporting DC cardioversion for persistent atrial fibrillation. Eur heart J 25: 144-150.
- Frick M, Frykman V, Jensen-Urstad M, Östergren J, Rosenqvist M (2011) Factors Predicting Success Rate and Recurrence of Atrial Fibrillation after First Electrical Cardioversion in Patients with Persistent Atrial Fibrillation. Exp Clin Cardiol 24: 238-244.
- Boodhoo L, Bordoli G, Mitchell AR, Lloyd G, Sulke N, et al. (2004) The safety and effectiveness of a nurse led cardioversion service under sedation. Heart 90: 1443-1446.
- Airaksinen KEJ, Hartikainen JEK (2013) Thromboembolic Complications After Cardioversion of Acute Atrial Fibrillation. J Am Coll Cardiol 62: 1187-1192.
- Weigner MJ, Caulfield TA, Danias PG, Silverman DI, Manning WJ (1997) Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med 126: 615-620.
- Stiell IG, Clement CM, Perry JJ (2010) Association of the Ottawa Aggressive Protocol with rapid discharge of emergency department patients with recent-onset atrial fibrillation or flutter. Can J Emerg Med 12: 181-191.
- https://www.havering.gov.uk/Documents/Equality-and-Diversity/Demographic_and_Diversity_Profile_of_Haverings_Population_Mar-14.pdf
- Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB (1978) Epidemiologic assessment of chronic atrial fibrillation and risk of stroke. Neurology 28: 973-977.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137: 263-272.
- Giralt-Steinhauer E, Roquer-Gonza´lez J (2012) CHA2DS2-VASc score and prognosis in ischemic strokes with atrial fibrillation. J Neurol 259: 745-751.
- Szulc M, Torbicki A (2001) Factors related to the early and late success of direct current cardioversion of chronic nonrheumatic atrial fibrillation: An echocardiographic study. Exp Clin Cardiol 6: 200-205.
- Gelder ICV, Lie KI (1996) Chronic Atrial Fibrillation: Success of Serial Cardioversion Therapy and Safety of Oral Anticoagulation. Arch Intern Med 156: 2585-2592.
- Sucu M, Davutoglu V, Ozer O (2009) Electrical Cardioversion. Ann Saudi Med 29: 201-206.
- Cardentey MC, Rosabal AM, Rivera TP (2014) Predictors of success of electrical cardioversion for atrial fibrillation. Cor Salud 6: 280-287.
- Sueda T, Nagata H, Orihashi K, Morita S, Okada K, et al. (1997) Efficacy of a Simple Left Atrial Procedure for Chronic Atrial Fibrillation in Mitral Valve Operations. Ann Thorac Surg 63: 1070-1075.
- Joseph T, Luck JC, Wolbrette DL, Patel HK, Naccarelli GV, et al. (1998) Drugs for Conversion of Atrial Fibrillation. Am Fam Physician 58: 471-480.
- Mabuchi N, Tsutamoto T, Maeda K, Kinoshita M (2000) Plasma Cardiac Natriuretic Peptides as Biochemical Markers of Recurrence of Atrial Fibrillation in Patients with Mild Congestive Heart Failure. Jpn Circ J 64: 765-771.
- Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, et al. (2008) Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 358: 2667-2677.
- Moreyra E, Finkelhor RS, Cebul RD (1995) Limitations of transesophageal echocardiography in the risk assessment of patients before nonanticoagulated cardioversion from atrial fibrillation and flutter: an analysis of pooled trials. Am Heart J 129: 71-75.
- Gallagher MM, Hennessy BJ, Edvardsson N, Hart CM, Shannon MS, et al. (2002) Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol 40: 926-933.
- Nuotio I, Hartikainen JEK, Grönberg T, Biancari F, Airaksinen J (2014) Time to Cardioversion for Acute Atrial Fibrillation and Thromboembolic Complications. JAMA 312: 647-649.
- Fatkin D, Kuchar DL, Thorburn CW, Feneley ML (1994) Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: Evidence for “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol 23: 307-316.
- Grönberg T, Nuotio I, Nikkinen M, Ylitalo A, Vasankari T, et al. (2013) Arrhythmic complications after electrical cardioversion of acute atrial fibrillation: The FinCV study. Europace 15: 1432-1435.
- Gramley F, Lorenzen J, Knackstedt C, Rana OR, Saygili E (2009) Age-related Atral Fibrosis. Age (Dordr) 31: 27-38.
- Essebag V, Hadjis T, Platt RW, Pilote L (2003) Amiodarone and the Risk of Bradyarrhythmia Requiring Permanent Pacemaker in Elderly Patients With Atrial Fibrillation and Prior Myocardial Infarction. J Am Coll Cardiol 41: 249-254.
- de Oliveira CV, Ciriello J (2003) Cardiovascular responses to hypocretin-1 in nucleus ambiguus of the ovariectomized female rat. Brain Res 986: 148-156.
- Resnekov L, McDonald L (1967) Complications in 220 Patients with Cardiac Dysrhythmias Treated by Phased Direct Current Shock, and Indications for Electroconversion. Br Heart J 29: 926-936.
- Goldbaum TS, Bacos JM, Lindsay L Jr (1986) Pulmonary edema following conversion of tachyarrhythmia. A case following burst atrial pacing. Chest 89: 465-467.
- (2014) National Institute for Health and Care Excellence. Clinical Guideline.
- Lip GY, Gregory YHL, Laroche C, Loachim PM, Rasmussen LH, et al. (2014) Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J 35: 3365-3376.
- Kirchhof P, Ammentorp B, Darius H, Caterina RD, Heuzey JYL, et al. (2014) Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events-European Registry in Atrial Fibrillation (PREFER in AF). Europace 16: 6-14.









