Nait Slimane N,Taieb M, Khiali R,Rabehi H, Bekhouche R and Bendjaballah A*
Department of General Surgery, Ain Taya Hospital, Algiers, Algeria
- *Corresponding Author:
- A. Bendjaballah
Department of General Surgery, Ain Taya
Hospital, Algiers, Algeria.
Tel: +213-551-764-640
E-mail: ali_bendjaballah@yahoo.fr
Received date: April 19, 2018 ; Accepted date: May 02, 2018; Published date: May 09, 2018
Citation: Slimane NN, Taieb M, Khiali R, Rabehi H, Bekhouche R, et al. (2018) A Huge Primary Hydatid Cyst of Uterus: A Case Report and Review of Literature. J Univer Surg. Vol.6 No.2:12
Keywords
Hydatid cyst; E. granulosus; Uterus; Primary cyst; Albendazole
Introduction
Hydatid cystic disease is a parasitic disease caused by the larval form of E.s granulosus [1,2]. Primary host of hydatid disease is the dog. Humans become an accidental intermediate host. The growth is slow and it may take several years before hydatid cyst become symptomatic. Hydatid cysts may be found in almost any part of the body, but are most often found in the liver (60%) and lungs (30%). The occurrence of hydatid disease is extremely low in genital organs, appearing with an incidence of 0.5%. Despite the plethora of publications concerning hydatid cyst, little is known about disease affecting genital organs especially the uterus and when it is primary This is a disease that has been known since antiquity and was described by Hippocrates with the particular term “liver filled with water”, followed by the famous Arabian physician Al-rahzes who wrote on hydatid cyst of the liver about one thousand years ago [3]. The life cycle of the parasite was acknowledged by Dew et al. [4].
Case Report
A 27 years old woman admitted in the surgical department for a voluminous uterine hydatid cyst associated with a chronic pelvic pain for 15 months of evolution. For the last five months it has become very large, which push the patient to think that she was pregnant. She had no history of any chronic illness. There is no history of hemoptysis or melena, or fever, no history of metrorrhagia. General and systemic examination of the patient was good. Examination of respiratory and cardiovascular systems revealed no abnormalities. Blood investigations were within normal values. The hydatid serology was strongly positive at 1/4500 (ELISA technique). The chest X-ray examination shown no hydatid localization at the both lungs and ECG was without abnormality. A beta HCG was negative. The abdomen was distended by the presence of an abdominal mass simulating a pregnancy of six months (Figure 1). The vaginal and rectal examination combined with abdominal palpation reveal a filling of the Douglas pouch by the imposing mass associated to a pain triggered by the mobilization of the cervix. Abdominal and pelvic ultrasound (Figure 2) objective a large cystic mass it was a hydatid cyst in a stage III of GHARBI’s classification “honeycomb”, the liver was free, no other abdominal localizations. The CT scan (Figure 3) showed a large cystic formation measuring 194 × 116 mm, multilocular developed from the posterior wall of the uterus probably hydatid origin. No anomalies found in the other part of the abdomen. The diagnosis of uterine hydatid cyst location was evident. The patient underwent surgery and the surgical approach was a median laparotomy under umbilicus which reveals an enormous mass of the posterior wall of the uterus. Protection of the peritoneal cavity and isolation of the uterus by fields soaked in oxygen peroxide at 10 volumes to avoid seeding and secondary Echinococcosis (Figure 4). The oxygen peroxide (H2O2) was used as scolicide. Repeated sterilization of the cystic cavity was done by H2O2, followed by total extraction of hydatid material (Figure 5). We use in this case laparoscopy camera to check the residual cavity which allowed us to detect several daughters’ vesicles that were not even possible before (Figure 6). This explains the recurrence observed in some patients. The resection of the projecting cyst wall was performed. She passed a good and quit post-operative period. She was discharged after 7 days. A long-term contraceptive treatment was prescribed for the patient to allow the myometrium to regenerate and restore its functions associated with an antiparasitic chemotherapy “Albendazole” to sterilize the residual cavity of the cyst and to ovoid recurrences. The patient was reviewed at one and three years after surgery and there was no recurrence. Hydatid serology was frankly diminished (1/320). The histological result was in favor of hydatid cyst of uterus (Figure 7).
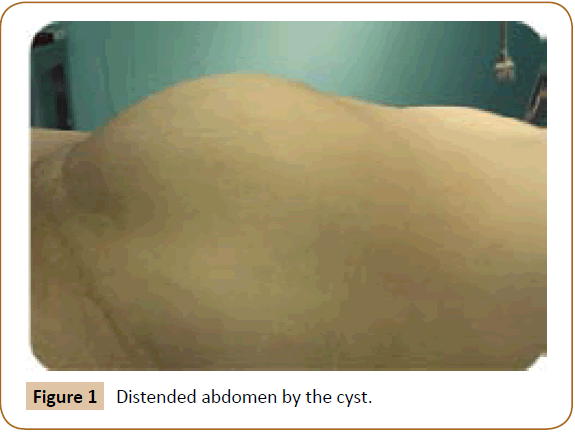
Figure 1: Distended abdomen by the cyst.
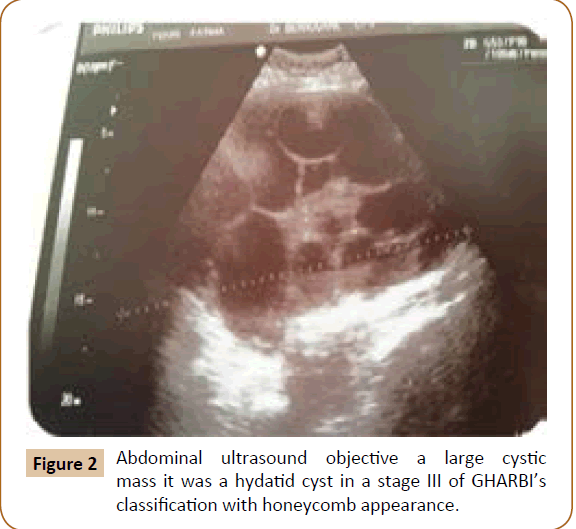
Figure 2: Abdominal ultrasound objective a large cystic mass it was a hydatid cyst in a stage III of GHARBI’s classification with honeycomb appearance.
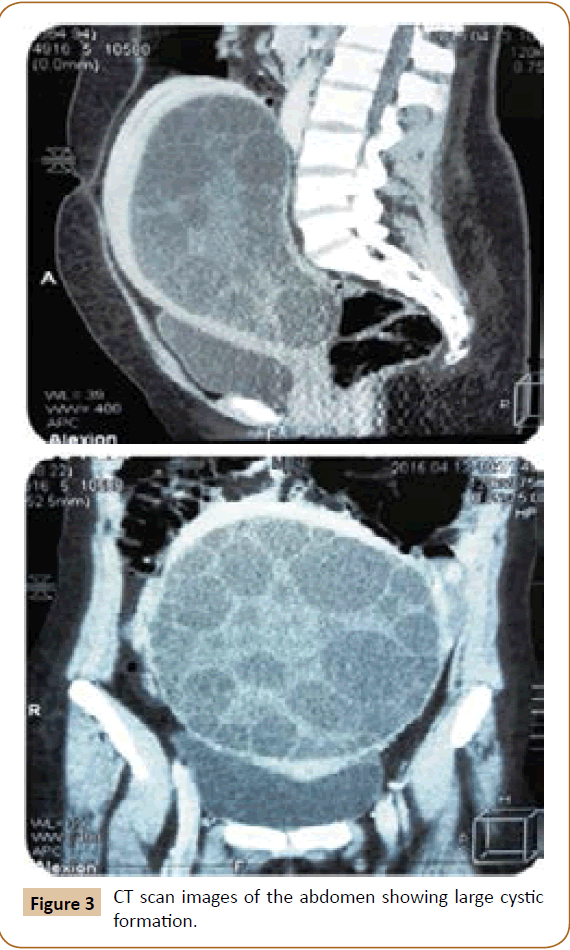
Figure 3: CT scan images of the abdomen showing large cystic formation.
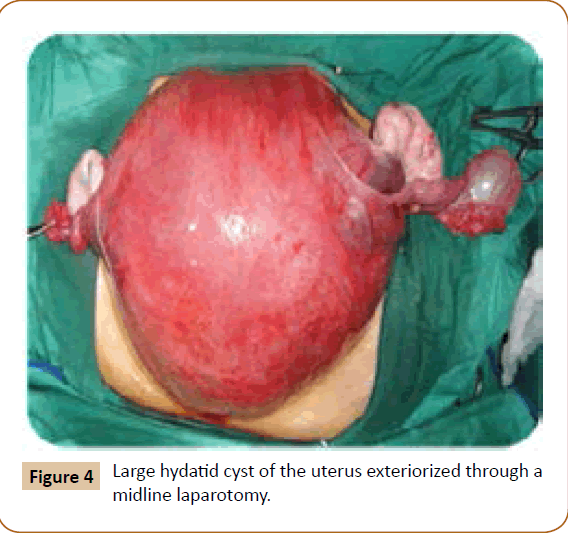
Figure 4: Large hydatid cyst of the uterus exteriorized through a midline laparotomy.
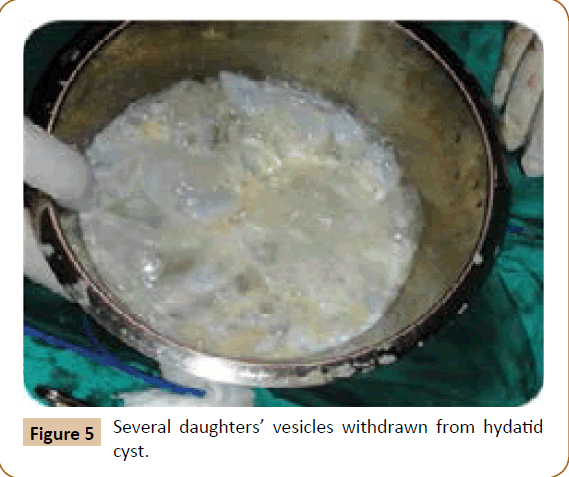
Figure 5: Several daughters’ vesicles withdrawn from hydatid cyst.
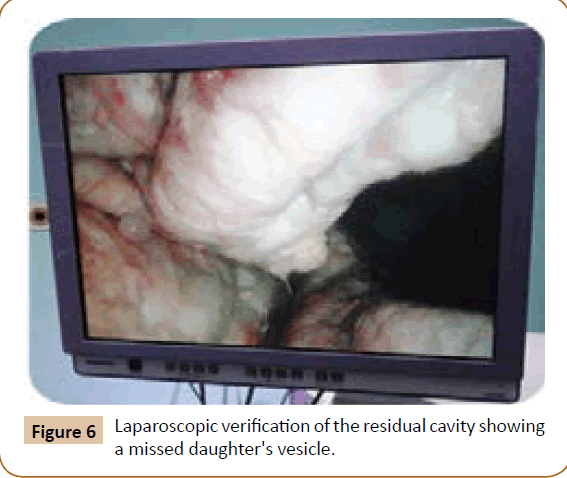
Figure 6: Laparoscopic verification of the residual cavity showing a missed daughter's vesicle.
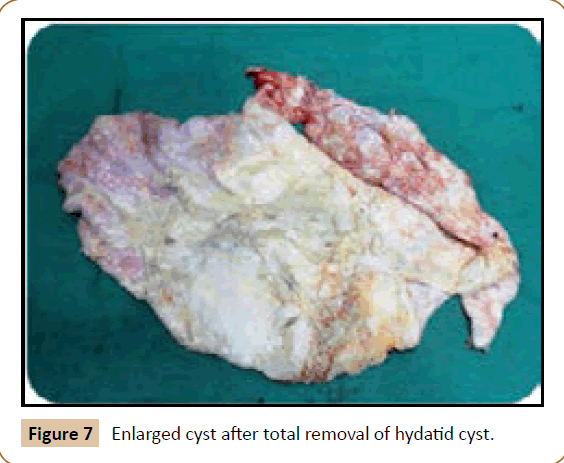
Figure 7: Enlarged cyst after total removal of hydatid cyst.
Discussion
Primary involvement of pelvic organs by E. granulosus is extremely rare and is almost always secondary to rupture into the abdominal cavity of hepatic cyst or cysts from other abdominal organs [5]. However primary pelvic Echinoccocus has been described and the disease appears to be exclusively confined to the genital organs [6]. Infestation by hydatid disease in humans occurs most commonly in the liver in about 70% of cases followed by the lung in about 20% of cases and the two organs can be affected simultaneously in about 15% of cases [7]. The involvement of pelvic region occurs in about 0.2 to 2% of cases [8]. Primary host of hydatid disease is the dog [9,10]. This parasite has a worldwide distribution. It is most prevalent in sheep- and cattle-raising countries. Human infection occurs from ingestion of material contaminated with dog (definitive host) feces containing tapeworm eggs, which hatch in the intestine; the larval embryos then penetrate the bowel wall and disseminate through the blood stream. A typical hydatid cyst is formed from its embryo. It consists of three layers of cystic membrane [11]. The outer layer “pericyst or adventitia” consists of fibrous tissue is grey in color. It is formed from the hot tissue as a result of chronic inflammatory reaction of the parasite. The pericyst usually increases in thickness as the cyst expands. Liver and spleen hydatid cyst have a thick pericyst, as compared to peritoneal hydatid cysts, in which the pericyst is extremely thin. In the lung and the brain there is no pericyst at all. With time, parts of pericyst may calcify and complete calcification may interrupt the nutrient an oxygen supply to the parasites and thus marks the death of the hydatid cyst. The parasite constitutes the laminated membrane (ectocyst) and the germinal layer (endocyst). The ectocyst has the appearance of the white of a hard-boiled egg. It is elastic, made up of gelatinous, chitinous material and when incised or ruptured, curls on itself, exposing the inner layer. The innermost germinal layer is cellular and consists of a number of nuclei embedded in a protoplasmic mass. It is a very thin, vital layer of the cyst and produces brood capsules with scolices, secretes hydatid fluid and form the outer layer. The cyst fluid is crystal clear and colorless with a specific gravity of 1,005 to 01,010, is slightly alkaline and is highly antigenic and toxic; contact with fluid can give rise to anaphylactic shock [12]. The larvae may come to rest and develop into hydatid cysts in any part of the body. Most of the embryos remain trapped in the liver, although the lungs, kidneys, spleen, central nervous system, and bone may become secondarily involved [13]. Hydatid cysts are usually solitary but may be multiple too. In our case, it was solitary, in the uterus only. The cyst usually grows slowly and occasionally reaches more in diameter. For our patient, it reached 19.4 cm × 11.6 cm in diameter and it becomes symptomatic with pelvic pain which have happen to be very common often requiring the use of antispasmodic by injection almost daily. Sonographic features of hepatic hydatid disease proposed are as follows [14]: simple cysts containing no internal architecture except sand, cysts with detached endocyst secondary to rupture, cysts with daughter cysts, matrix (echogenic material between the daughter cysts) or both, and densely calcified masses. In this case this solitary hydatid cyst is most probably primary not secondary because of absence of liver, lung or other locations and it was classified as stage III of GHARBI’s classification [15]. The preoperative ultrasonography and CT scan examination of the abdomen were carried out to explore the nature of the cystic mass and define the relations of this one with the adjacent organs. This two imaging investigations (CT scan and US) have been well established techniques in the evaluation of hydatid disease and in the follow-up [16]. On the CT scan the uterus was found enlarged with an anterior posterior diameter of 194 mm. In the uterus, a 174-102 mm-diameter well-defined cystic mass was found with innumerous small cysts or septae inside it. The endometrium could not be outlined. Myometrium was thinner. The cervix was of 25 mm in diameter. The ovaries have been well individualized and normal. No adnexal cyst was detected. The resolution of CT scan is very excellent in the evaluation of hydatid cyst and detecting calcifications in the cyst wall [17]. CT scan is to be superior to ultrasound examination in especially defining extent of abdominal cyst [18]. The involvement of female genital tract in hydatid disease is extremely rare but the most important factor in its diagnosis is the awareness of the possibility of hydatid disease especially in endemic area [19]. Imaging investigations (US and CT scan) appears to be helpful for detection of unusual location of hydatid disease. Magnetic resonance imaging (MRI) signal characteristics have also reported [20] undoubtedly; the experience of the radiologist particularly in an endemic area will promote a higher index of suspicion leading to the diagnosis [18]. Surgery is still the mainstay of treatment of hydatid cyst. Several surgical approaches from simple drainage to radical liver resection have been described for hydatid cysts. Laparoscopic interventions are usually limited to cystotomy and drainage [21,22]. For our patient the diagnosis was more or less easy because there was a single voluminous cystic formation at the level of the uterus and the hydatid etiology was no doubt. There were some elements which oriented our diagnosis: the duration of symptomatology (several months before) the patient came from endemic area, ultrasound and CT scan findings (one primary solitary hydatid cyst of the posterior wall of uterus), and finally the serology of hydatid cyst (ELISA) was highly positive. It has been shown that in endemic areas the possibility of hydatid disease should be considered in the differential diagnosis of any mass or growing tumor in the female pelvis [23]. However Hydatidiform mole, missed abortion with hydrophic degeneration, and fibroid uterus with cystic degeneration are the differential diagnoses of this case of uterine hydatid cyst. In hydatidiform mole, numerous small, cystic, fluid containing spaces are scattered throughout the soft tissue mass filling the uterine cavity. Doppler evaluation of trophoblastic tissue reveals a lowimpedance, high-flow state. The incidence of theca lutein cysts in trophoblastic disease is 20 to 50%. Then there is markedly elevated level of serum β-hCG. On Doppler examination, the walls and septae of the cyst showed very poor vascularity. There was no ovarian cyst, and the serum β-hCG of our patient was negative. Missed abortion with hydrophic degeneration may look the same, but low serum β-hCG level and low-flow; higher impedance on Doppler evaluation will exclude it. Leiomyomas with cystic degeneration will have similar Doppler state, and it will be highly attenuating [24]. All hydatid cysts should be removed surgically if the patient’s general condition permits. Surgery is accepted as the treatment of choice in Echinococcal disease, although recent reports describe success with percutaneous drainage [25,26]. Surgical removal is the usual treatment in women with pelvic hydatid disease [27]. The World Health organization has outlined the treatment guidelines for hydatid cysts. Surgery is the treatment of choice for all patients with symptomatic disease and who are fit for surgery [28]. The total surgical removal of all the cysts was applied for our patient and there was no spillage of cyst content at operation. In case of incomplete surgical removal or there was spillage of the cyst content in the abdominal cavity, oral medical treatment “Albendazole” have proved to be quite effective in this situation and should be started in the early post-operative period with a dose of 400 mg twice daily for 28 days followed by an another course of 28 days separated by 15 days of rest [16,29]. We use in this case laparoscopy camera to check the residual cavity which allowed us to detect several daughters’ vesicles embedded in folds of this cavity that were not even possible before (Figure 6). This explains the recurrence observed in some cases. The medical treatment has been established based on Albendazole 400 mg/ day. I want to point out in this work that the Council of ethics in Algeria adopted for the first time the use of Albendazole in treatment of the hydatid cyst. Medical treatment is an alternative. Ultrasound has been used to monitor the course of medical therapy in patients with abdominal hydatid disease [30]. These changes were noted in the resolution of the disease were a gradual reduction in cyst size (43%), membrane detachment (30%), progressive increase in echogenicity of the cyst cavity (12%), and wall calcification (6%). No change was identified in 26% of the patients [26].
Several surgical approaches from simple drainage to radical liver resection have been described for hydatid cysts. Laparoscopic interventions are usually limited to cystotomy and drainage [21,22]. The laparoscopic treatment of hydatid cysts with advances and increasing experience in laparoscopic surgery, many more attempts have made to offer the advantage of such a procedure to this patient [31]. Surgery is the gold standard and primary treatment, with a variety of techniques based on the principles of eradication and elimination of recurrence while avoiding spillage [32]. Laparoscopic treatment of hydatid cyst liver has been increasingly popular parallel to the progress in laparoscopic surgery [33]. However, controversies about the role of laparoscopy in the management of hepatic hydatid cyst have not been resolved, because of limited experience worldwide. These controversies included selection of patient, surgical technique and follow up [34]. Laparoscopic pericystectomy combines the advantages of radical surgical resection with those of minimally invasive surgery [21,22]. For good cosmetic appearance effort aiming to reduce incision of laparoscopic surgery has been thought recently. For this purpose Single Incision Laparoscopic Surgery (SILS) has gained momentum in different fields of surgical practice [35,36].
Conclusion
This is a rare case of primary and solitary hydatid cyst in the uterus, which was diagnosed preoperatively by ultrasonography and CT scan. Detection of atypical localization is made easier with imaging procedures. The location of hydatid cyst in the genital tract is rare, and its presence in the uterus is extremely rare. A careful sonographic examination of the pelvic masses should be carried out to avoid wrong diagnosis. Laparoscopic treatment of hydatid cysts is now well established around the world with very good results and should be used whenever possible. This disease constitutes a serious problem of public health in the endemic countries. In order to interrupt the cycle of transmission of hydatid cyst, public health measures should be implemented to eradicate the disease by the removal of infected animals.
22961
References
- Basgul A, Kavak ZN, Gokaslan H, Kullu S (2002) Hydatid cyst of the uterus. Infect Dis Obstet Gynecol 10: 67-70.
- Sahin E, Nayki U, Sadik S, Oztekin O, Nayki C, et al. (2005) Abdominal and pelvic hydatid disease during pregnancy. Arch Gynecol Obstet 273: 58-59.
- Katan YB (1977) Intrabiliary rupture of hydatid cyst of liver. Ann Rev Coll Surg Engl 59: 108-114.
- Abu-Eshy S (1999) Hydatid cyst associated with pregnancy: A case report and review of the literature. Ann Saudi Med 19: 130-131.
- Rahman MS, Rahman J, Lysikiewicz A (1982) Obstetric and gynaecological presentations of hydatid disease. Br J Obstet Gynaecol 89: 665-670.
- Scheye T, Aufauvre B, Vanneuville G, Vincent G, Goddon R, et al. (1994) Abdominal cystic lymphangiomas in children. J Chir (Paris) 131: 27-33.
- Azhar H (1977) Primary echinococcal infection of the ovary. Br J Obstet Gynaecol 84:633
- Kir A, Baran E (1995) Simultaneous operation for hydatid cyst of right lung and liver. Thorac Cardiovasc Surg 43: 62-64.
- Gueddama F, Chemmen L, Lebbi I, Koubaa A, Benzined T, et al. (1990) Intrauterine hydatidosis: A case report. J Gynaecol Obstet Biol Report (Paris) 19: 725-727.
- Daifalah I (2001) Hydatid cyst of the uterine cervix. Biomed papers 145: 77-78.
- Milicevic M (1994) Hydatid disease. in: Blumgart LH Surgery of the Liver and Biliary Tract (2nd ed). New York: 1121-1150.
- Mehra BR, Thawait AP, Gupta DO, Narang RR (2007) Giant abdominal hydatid cyst masquerading as ovarian malignancy. Singapore Med J 48: e284-286.
- Withers CE, Wilson SR (1998) The liver. In: Rumack CM, Wilson SR and Charboneau JW Diagnostic Ultrasound (2nd ed). St. Louis: 87-154.
- Lewall DB, McCorkell SJ (1985) Hepatic Echinococcal cysts: Sonographic appearance and classification. Radiology 155: 773-775.
- Gharbi HA, Hassine W, Brauner MW, Dupuch K (1981) Ultrasound examination of hydatid liver. Radiology 139: 459-463.
- Moussa AM, Mehrez MG, Muhtaseb SA, Al-Mudala DS, Kamel SM (1987) Disseminated pelvic hydatidosis presenting as ovarian carcinomatosis: Successful post-operative treatment with mebendazole. Int J Gynaecol Obstet 25: 473-478.
- Idris M, Ahmad A, Afridi SJ (1999) Imaging spectrum of cystic echinococcosis. Journal of Ayub Medical College 11: 10-13.
- Adewunmi OA, Basilingappa HM (2004) Primary ovarian hydatid cyst in the Kingdom of Saudi Arabia. Saudi Med J 25: 1697-1700.
- Arora M, Gupta CR, Jindal S, Kapoor N (2005) An unusual case of hydatid cyst of broad ligament. Journal, Indian Academy of Clinical Medicine 6: 86-87.
- Sing S, Gibicote SV (2001) Magnetic resonance imaging signal characteristics in hydatid cyst. Australas Radiol 45: 128-133.
- Kopan M, Yavuz N, Kapan S, Polat S, Goksy E (2004) Totally laparoscopic pericystectomy hepatic hydatid disease. J Laparoendosc Adv Surg Tech A 14: 107-109.
- Khoury G, Abiad F, Geugia T, Nabout G, Jabbour S (2000) Laparoscopic treatment of hydatid cyst of the liver and the spleen. Surg Endosc 14: 243-245.
- Fazili A, Wani NA, Khan TS, Mir AR (2002)Hydatidosis: Rare presentations. JK Practioner 9: 252-253.
- Callen PW (1994) Ultrasound evaluation of gestational trophoblastic neoplasia. In: Callen PW Ultrasonography in obstetrics and gynecology (3rd ed) Philadelphia: WB Saunders Co.: 615-624.
- Mueller PR, Dawson SL, Ferrucci Jr JT, Nardi GL (1985) Hepatic echinococcal cyst: Successful percutaneous drainage Radiology 155: 627-628.
- Bret PM, Fond A, Bretagnolle M, Valette PJ, Thiesse P, et al. (1988) Percutaneous aspiration and drainage of hydatid disease of the liver. Radiology 168: 617-620.
- Okumus Y, Tayyar M, Patiroglu T, Aygen E (1994) Uterine hydatid cyst. Int J Gynaecol Obstet 45: 51-53.
- World Health Organization (1996) Guidelines for treatment of cystic and alveolar echniococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ 74: 231-242.
- Keshmiri M, Baharvahdat H, Fattahi SH, Davachi B, Dabiri RH, et al. (2001) Albendazole versus placebo in treatment of echinococcosis. Trans Res Soc Trop Med Hyg 95: 190-194.
- Bezzi M, Teggi A, De Rosa F, Capozzi A, Tucci G, et al. (1987) Abdominal hydatid disease: Ultrasound findings during medical treatment. Radiology 162: 91-95.
- Gorad K, Rayate N, Oswal K, Krishna A, Deshmukh A, et al. (2011) Laparoscopic removal of pelvic hydatid cysts in young female: A case report. Minim Invasive Surg.
- Dervenis C, Delis S, Avgerinos C, Madariage J, Milicevic M (2005) Changing concepts in the management of liver hydatid disease. J Gastrointest Surg 9: 869-877.
- Ertem M, Karahasanoglu T, Yavus N, Erguney S (2002) Laparoscopiclly treated liver hydatid cysts. Arch surg 137: 1170-1173.
- Memon MR, Channa SM, Jamro BU (2012) Role of laparoscopy in the management of hydatid cyst of liver. Rawal Med J 37: 322-324.
- Barbaros U, Sumer A, Tunca F, Gozkun O, Dimerel T, et al. (2010) Our early experiences with single incision laparoscopic surgery: The first 32 patients. Surg Laparosc Endosc Percutan Tech 20: 306-311.
- Xiu-Jun C, Zhi-Yi Z, Xiao L, Hong Y, Fan WY, et al. (2010) Single incision laparoscopic surgery: A case report. Chin Med J 123: 269-220.












