Keywords
Ex vivo testicle perfusion; Whole testicle cryopreservation; Gonadotoxic cancer therapy; Fertility preservation
Introduction
Testicular cryopreservation aims at the preservation of fertility in children and prepubertal boys, subjected to gonadotoxic anti-cancer therapy, hematopoietic stem cells transplantation and other reasons of intensive chemotherapy. Further indications could differ from country to another, based on the available medical regulations [1,2].
Testicular cryopreservation can be performed using the slow freezing technique, where a programmable controlled temperature change can be applied, or the Vitrification technique, where the testicular tissue is equilibrated with suitable concentrations of cryoprotectants and directly plunged into liquid nitrogen [3,4].
Recently, the technique of cryopreservation has been tried with animals and humans, applying both slow freezing and Vitrification techniques, with the results of Vitrification are comparable to those of slow freezing [5,6].
Ex Vivo Testicle Perfusion
In the present technique, the testicle is to be removed by an open surgical approach (scrotal or inguinal), where the vascular pedicle of the testicle is accessed. The testicular artery is approached as proximal as possible, and catheterized after giving off its branches. Similarly, the testicular vein is approached as distal as possible, and catheterized before receiving its tributaries.
Now, the testicle can be surgically removed, while both the vascular input and output are catheterized, however, before removal, in vivo perfusion through the catheters is to be performed for few minutes, to avoid ischemia and any risk of microthrombi formation.
The arterial and venous catheters are connected to a circuit of perfusion, composed of the following (Figure 1):
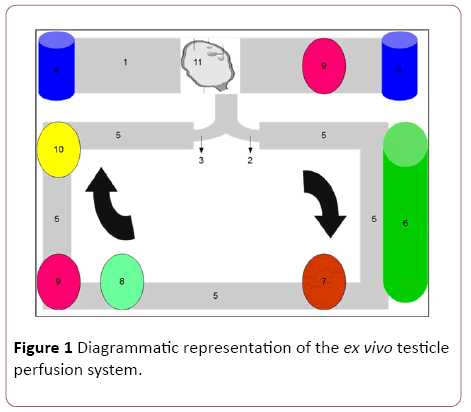
Figure 1: Diagrammatic representation of the ex vivo testicle perfusion system.
1. A box to enclose the testicle and can be used as a sealed cryovial.
2. The box enclusing the testicle can work as a sealed cryovial, which allows the passing of the perfusion input and output catheters during perfusion, and the complete sealing during liquid nitrogen storage
3. The circuit itself is made of perfusion tubes, connected to the arterial and venous catheters.
4. Reservoirs to introduce and remove the perfusates (perfusion supplemented medium, cryopreservation and warming solutions)
5. A pulsatile or continuous flow centrifugal pump, providing flow equivalent to the estimated physiological testicular artery flow
6. A set of leukocytes and cytokines filters, to minimize the inflammatory injury of ischemia and reperfusion
7. A temperature adjustor that can control the temperature of the perfusates.
8. A gas exchanger to remove CO2 and provide O2, to maintain these gases in the perfusates at the physiological levels.
The introduced procedure start with the surgical retrieval of the testicle, where vascular catheterization and immediate perfusion begins. The used perfusates can vary according to the protocol used, for instance, minimal essential medium (MEM) supplemented with human serum albumen (HSA) or human tubal fluid medium supplemented with serum substitute supplement (SSS). Further supplementations could be considered according to the used protocol, such as ascorbic acid, antioxidants, hormones, growth factors, antibiotics, heparin … etc.
The immediate and continuous perfusion minimizes the risk of microthrombi formation, however, thrombolytic medications could be supplemented to the perfusates, in order to dissolve any formed microthrombi. At this stage, the testicle has not manifested significant ischemia or oxygen or nutrient deprivation, and the vascular bed is clearly accessible.
Following a short period of ex vivo perfusion, the cryopreservation solution (either for slow freezing or for Vitrification) can be introduced into the circuit to simultaneously fill the cleaned vascular bed of the testicle and the plastic box around the graft. This ability, together with the presence of temperature adjustors, allow the application of the cryopreservation or Vitrification protocol of interest, where at the end, the graft can be stored in liquid nitrogen. These abilities of the system allow, as well, the application of the warming protocols, where the graft can be further perfused till the surgical transplantation, minimizing the ischemic reperfusion injury.
The Expected Advantage
The fertility restoration through testicular tissue cryopreservation has been tested and succeeded in the animal models as well as in human, and accordingly has been clinically applied. However, little is known about the safety aspects and the quality of the offspring generations [6]. In the present innovation, a new strategy for testicular cryopreservation is introduced. Whether or not it could provide better and or safer clinical results, this could be the addressed question for future studies.
The expected advantage of the proposed strategy and system over the currently applied techniques of testicular tissue or sperm cryopreservation would be the potential of the unrestricted preservation of fertility, the dual preservation of fertility and endocrine testicular functions (Table 1), and the preservation of the physiological way of fertilization, i.e. the sexual intercourse, without the application of assisted reproduction technology, which, at least, would have a significant psychological impact.
| Hormone |
Origin |
Regulation |
Action |
| Testosterone |
Leydig Cells |
Gonadotropin releasing hormone from the hypothalamus causes luteinizing hormone secretion from the pituitary gland, which stimulates the Leydig cells |
The control and maintenance of the growth and functions of thereproductive organs, libido and spermatogenesis |
| Inhibin |
Sertoli Cells |
Gonadotropin releasing hormone from the hypothalamus causes follicle stimulating hormone (FSH) secretion from the pituitary gland, which stimulates the Sertoli cells |
Pituitary feedback inhibition of FSH |
| Oestradiol |
Sertoli Cells |
Gonadotropin releasing hormone from the hypothalamus causes follicle stimulating hormone secretion from the pituitary gland, which stimulates the Sertoli cells |
Produced by testosterone metabolism and may prevent the apopotosis of male germ cells |
Table 1: Hormones produced by the testicles.
Moreover, the system allows the ex vivo testicle perfusion, where high doses of supportive elements and or medications could be supplemented to correct and or improve the testicular functions. While this manuscript presents the innovative idea in its theoretical form, the author is willing to cooperate for further experimental realization and testing of the strategy and the system.
Conflicts of Interest
The intellectual properties and the system included in this manuscript belong solely to the author. All rights are preserved solely for the author. Reproduction or use of any of the included intellectual properties requires the written permission of the author. No funding was provided for the development of this work. The author welcomes funding cooperation for experimental and clinical studies.
18954
References
- Donnez J, Dolmans MM, Martinez-Madrid B, Demylle D, Van Langendonckt A (2005) The role of cryopreservation for women prior to treatment of malignancy. Curr Opin Obstet Gynecol 17: 333-338.
- Johannes O, Nouri K, Stögbauer L, Fischer EM, Lipovac M, et al. (2010) Ovarian tissue cryopreservation for non-malignant indications. ArchGynecolObstetr 281: 735-739.
- Gangrade BK (2013) Cryopreservation of testicular and epididymal sperm: Techniques and clinical outcomes of assisted conception. Clinics (Sao Paulo) 68: 131-140.
- Mohamed SAM (2015) Slow cryopreservation is not superior to vitrification in human spermatozoa:An experimental controlled study. Int JReprod BioMed (Iran J Reprod Med) 13: 633-644.
- Honaramooz A (2012) Cryopreservation of testicular tissue.Curr Front Cryobiol.
- Onofre J, Baert Y, Faes K, Goossens E (2016) Cryopreservation of testicular tissue or testicular cell suspensions: A pivotal step in fertility preservation. Hum Reprod Update 22: 744-761.






