Keywords
Normative; Maha-tita; Bitterness; Andrographis paniculata; Acanthaceae; Creat; Kirayat; Kalmegh; Nilavembu; Nigerian; Asian; Characteristics.
Introduction
“Maha-tita” in Hindi means “king of bitters”. “Creat” is the English name for the herb Andrographis paniculata that belongs to the family: Acanthaceae. The herb is exceedingly bitter, and has been in use for many centuries in Asia, where it is regarded as the “King of Bitters” [1, 2]. It grows erect to a height of over one meter in moist shady habitats. It has ovate, pinnate or lanceolate leaves of various sizes. The flowers have minute white petals bearing purplish spots. The stem is deep green, with diameter ranging from 2 mm to 6 mm or more. The flowers give rise to oblong capsules bearing numerous, minute brown seeds. The plant reproduces by seeds, and is widely distributed in tropical Asia. Its centre of origin and diversity is thought to be Sri Lanka and south India, but the herb is found in north India, China, and the entire Southeast Asia. Unlike other species of Andrographis, the herb called “Kirayat” in Hindi, “Kalmegh” in Bengali or “Nilavembu” in Tamil, occurs quite commonly in all of the India subcontinent, which accounts for its widespread use since ancient times against a variety of disorders, in both Ayurvedic and Chinese Medicine [1-3]. The aqueous extract has activity against Salmonella and Candida, and is reported to be antihepatitic, antihepatotoxic, antibiotic, antimalarial, anti-HIV, antithrombogenic, antiinflammatory and antipyretic. The herb is also famed as an immunostimulant [4]. The principal agent, andrographolide, first isolated by Gorter in 1911, is mostly extracted from the leaves [2, 5]. It is an intensely bitter, water-soluble lactone that protects rats against carbon tetrachloride induced hepatotoxicity [2, 3]. The LD50 in male mice is 11.46gm/kg. Andrographolide has been shown to inhibit in vitro proliferation of different tumor cell lines. The compound is thought to exert direct anticancer activity by arresting cell cycle at G0/G1 phase; by inducing cell cycle inhibitory protein - p27; and decreasing the expression of cyclin dependent kinase 4 (CDK4) [1-3]. Immunostimulatory activity of andrographolide is evidenced by increased proliferation of lymphocytes and production of interleukin 2 [1, 2]. Andrographolide also enhances the production of tumor necrosis factor; and the expression of CD marker, resulting in increased cytotoxic activity of lymphocytes against cancer cells [1]. The herb which bitterness and other properties are known to vary with habitat [1-3], was introduced to NIPRD, Nigeria, from India in the late1990s. It has since then been in cultivation in the Institute’s gardens for purposes of drug research and development. The seeds are sown early in the rainy season (March). The herb begins to flower as from about August or September. In this present paper the “bitterness value” and some basic features of the Nigerian grown Andrographis paniculata are reported.
Macroscopic And Sensory Examinations Of The Fresh Plant
These were carried as described in the WHO manual on quality control of medicinal plant materials [6] on plants obtained from NIPRD’s botanical gardens. The shape, size, colour, odor and taste of the aerial parts were examined. Events and aspects related to life cycle, habits and habitat were noted.
Treatment And Sampling Of The Dried Aerial Parts
The aerial parts of herb harvested during the months of September and October were air-dried in a well ventilated shade in the Institute for drying medicinal plant materials, and subsequently comminuted to coarse powder with a grinding machine. The procedure was as described by WHO [6] as follows: Three (3) original samples from each batch were combined into a pooled sample and subsequently used to prepare the average sample. The average sample was prepared by “quartering” the pooled sample as follows. Each pooled sample was mixed thoroughly, and constituted into a square-shaped heap. The heap was then divided diagonally into 4 equal parts. Any 2 diagonally opposite parts were taken and mixed carefully. This step was repeated as necessary until the required quantity of sample was obtained. Any material remaining was returned to the batch. The final samples were obtained from an average sample by quartering, as described above. This means that an average sample gave rise to 4 final samples. Each final sample was divided into 2 portions. One portion was retained as reference material, while the other was tested in duplicate or triplicate.
Determination Of Bitterness Value
The bitterness of the herb was determined by the method of WHO [6] which compares the threshold bitter concentration (TBC) of an extract of the herb with the TBC of a dilute solution of quinine hydrochloride. The bitterness value is expressed in units equivalent to the bitterness of a solution containing 1 g of quinine hydrochloride in 2000 ml. The method is identical to that described in the European Pharmacopoeia [7] as recently used by Meyer and coworkers [8]. The bitterness value is calculated as follows:
BITTERNESS VALUE in units per g = (2000 x C)/ (A x B), where:
A = the concentration of the herbal stock solution (Sh) in (mg/ml).
B = the volume of (Sh (in ml) in the tube with the threshold bitter concentration.
C = the quantity of quinine hydrochloride (in mg) in the tube with the threshold bitter concentration.
Physicochemical Tests
The following tests, briefly described, were carried out on the extracts as per WHO [1]:
Loss on drying (LOD)
This was carried out using a minimum of 0.5 – 1.0 g of material. Drying was effected in a gravityconvention oven (Lindberg/Blue M) maintained at 105-110 °C. The results are expressed as a range or as mean ± standard deviation. The LOD results were validated by concurrent determination of the LOD of CuSO4 crystals, which was 36.43 %w/w.
Total ash (TA) and Acid insoluble ash (AIA)
These values were determined using a minimum of 0.5 – 1.0 g of material and a furnace (Vecstar Furnace) heated gradually to the ignition temperature of 650 - 700 °C. The process was repeated until at least two consecutive constant weights were obtained. The results are expressed as a range or as a mean value ± standard deviation. The TA results were validated by concurrent determination of the TA of paracetamol BP, which was less than 0.01 % w/w.
Water extractability by hot extraction
About 4.0 g of coarsely powdered, air-dried sample is accurately transferred into a glass-stoppered, 250- ml, reflux conical flask, followed by 100 ml of water. The flask is weighed with the contents, and the weight is recorded (W1). The flask is well shaken, and allowed to stand for 1 hour. Subsequently a reflux condenser was attached to the flask, and boiled for 1 hour; then the flask is cooled and weighed again with the contents - the weight (W2) is recorded, and readjusted to (W1) with water. The flask is again well shaken, and the contents rapidly filtered through a dry filter paper. By means a pipette, 25.00 ml of the filtrate is transferred to a previously dried and tarred glass dish. The dish is then gently evaporated to dryness on a hot plate. Subsequently, the dish is dried at 105ºC for 6 hours, cooled in a desiccator for 30 minutes, and weighed. The water extractable matter is calculated as %w/w of the air-dried sample.
Phytochemical Tests
The following tests as described by Harborne [9] and Onwukaeme and coworkers [10] were carried out on the herb or aqueous extract as follows:
Fehling's test for glycosides
To about 10 mg of the extract in test-tube was added 2 ml of water, followed by 0.2 ml of 0.1 M HCl, and warming – to effect hydrolysis of any glycosides. This was followed by the addition of 1ml each of Fehling's solutions A and B, with shaking under a bath for 10 minutes. A brick-red precipitate indicates reducing sugar.
Frothing test for saponins
A pinch of the aqueous extract was added to 5 ml of water and warmed until dissolved. The solution was subsequently shaken vigorously to generate froth, and then allowed to stand. A rich froth persisting after 10 indicates the presence of saponins.
Borntrager's test for anthraquinone derivatives
About 100 mg of air-dried herb is extracted with 5 ml of chloroform by shaking and warming under a bath. To about 2 ml of the supernatant, 1ml of dilute 10 % v/v ammonia solution was added, followed by shaking. A pink or red colour in the aqueous layer indicates the presence of anthraquinone derivatives.
Ferric chloride solution test for tannins
A pinch of the aqueous extract was vigorously shaken with 3 ml of warm water until dissolved. This was followed by 1 ml of 15 % ferric chloride test solution. A blue-green coloration indicates tannins.
Dragendorff's tests for alkaloids
About 20 mg of the air-dried herb was extracted with 20 ml of methanol by shaking and heating under a bath. The extract was subsequently filtered and allowed to cool. Each 2 ml of the filtrate in a test-tube was treated with the Dragendorff's reagent. The development of a yellow precipitate indicates alkaloids.
Thin Layer Chromatography (Tlc)
Florescent, precoated plates were used for both the normal and reverse phase TLC. The normal phase utilized silica K6, and hexane: ethylacetate: methanol (4:4:1) as mobile phase; while the reverse phase utilized KC18 plate, and methanol: water (80:20). Solutions of analytes were prepared and applied as follows: To 1 mg of the analyte, 2 drops of ethanol were added and mixed well (~1%w/v solution). The plates used were 5 cm wide x 20 cm long. With a ruler and a pencil, a distance of 5 mm was measured from the bottom of the plate, and a line of origin was lightly drawn across the plate, without disturbing the adsorbent. The analyte was applied to the origin as a 1 µl droplet. The spot was allowed to dry. Subsequently, the plate was developed in a developing tank saturated with the vapour of the solvent system to be used as mobile phase. The level of the solvent in the tank was adjusted to a level 2 to 3 mm below the line of origin on the plate. The plate was considered developed when the solvent front reached a predetermined line, not less than 5 mm below the top of the plate. The air-dried plate is visualized using a viewing cabinet (CAMMAG) and a UVlamp (CAMMAG – equipped to emit light at 254 or 366 nm). The resulting chromatogram is photographed or drawn to scale.
Results
Results of the macroscopic and sensory examinations are shown Figure 1 and Table 1. Those for the determination of bitterness value are shown in Table 2, while those for the physicochemical determinations are shown in Table 3. The phytochemical profile of the herb is shown in Table 4, while the attempt to provide TLC fingerprints for the herb is indicated in Figure 2.
Volunteer
( ?or ?) |
[C] mg of Sq in tube with TBC |
[A] mg/ml
of Sh |
[B] ml of Sh in
Tube with TBC |
Bitterness Value: units/g
(2000 x C)/ (A x B) |
| SJA± male |
0.046 |
0.01 |
6.0 |
1.53 x 103 |
| MJS ± male |
> 0.058 limit |
0.01 |
- |
- |
| NKO ± male |
0.052 |
0.01 |
> limit of 10 |
- |
| OOD ± male |
0.044 |
0.01 |
6.0 |
1.47 x 103 |
| ATA ± male |
0.050 |
0.01 |
7.0 |
1.43 x 103 |
| DAE ± male |
0.046 |
0.01 |
2.0 |
4.60 x 103 |
| CHS ± male |
0.046 |
0.01 |
7.0 |
1.31 x 103 |
| Mean ± SD |
0.043 ± 0.003a |
- |
- |
2.07± 1.42 b x 103 |
| MOI ± female |
0.046 |
0.01 |
5.0 |
1.84 x 103 |
| OBS ± female |
0.044 |
0.01 |
4.0 |
2.60 x 103 |
| CSO ± female |
0.048 |
0.01 |
2.0 |
4.80 x 103 |
| RHB ± female |
0.054 |
0.01 |
2.0 |
5.40 x 103 |
| EOO ± female |
0.052 |
0.01 |
8.0 |
1.30 x 103 |
| BSA ± female |
0.052 |
0.01 |
2.0 |
5.20 x 103 |
| Mean ± SD |
0.049 ± 0.004 a |
- |
- |
3.52 ± 1.82 b x 103 |
Footnote to Table 1: (a) indicates that the difference between the means is statistically significant at P < 0.05.This suggests that the males were more sensitive than females to the bitterness of quinine hydrochloride. Although the males appear less sensitive to the bitterness of Andrographis paniculata, in that the mean bitterness value for males appeared to be lower than that of the female, the difference denoted by (b) is however not statistically significant at P = 0.05 (two-tail, 10 degrees of freedom). The mean bitterness value for both sexes is 2.86 ± 1.74 x 103.The volunteer denoted (*), aged 42, had cold at the time of the experiment. If the result of the volunteer (1.30 x 103) is set aside, the female mean value becomes 3.97 ± 1.63c x 103. Similarly, if the most extreme male result (4.60 x 103) is set aside, the male mean value becomes 1.44 ± 0.09 c x 103. In that scenario the sex difference denoted by (c) becomes statistically significant at P < 0.05 (two-tail, 8 degrees of freedom).
Table 1: Determination of the bitterness value of Andrographis paniculata.
Parameter
(%w/w) |
Material from NIPRD |
Material Reported by WHO [11] |
| Description |
Dry, dark green aerial parts; practically odorless or faint and
characteristic aroma. |
- |
| Loss on Drying |
10.64 (7) |
Not more than 10% w/w |
| Total Ash |
14.10 (7) |
- |
Water
Extractability |
30.37 (8) |
Not less than 18 % w/w |
Acid Insoluble
Ash |
1.00 (2) |
Not more than 2 % w/w |
Footnote to Table 2: The numbers in parentheses indicate the numbers of samples/ determinations carried out in duplicates or triplicates.
Table 2: Physicochemical properties of aerial parts of Andrographis paniculata
| Test |
Observation |
Inference |
| Fehling's test for glycosides |
Red ppt. results |
Glycosides are present |
| Frothing test for saponins |
Copious froth results |
Saponins are present |
| Test for anthraquinones |
No apparent change |
Anthraquinones may be absent |
| FeCl3 test for tannins |
Blue-black ppt. results |
Tannins are present |
| Test for alkaloids |
Yellow ppt. results with Dragendorff as reagent. |
Alkaloids are present |
Footnote to Table 3: The likely absence of anthraquinones in Andrographis is favorable outcome since the material is intended for internal use.
Table 3: Inference from tests for phytochemical constituents of Andrographis paniculata
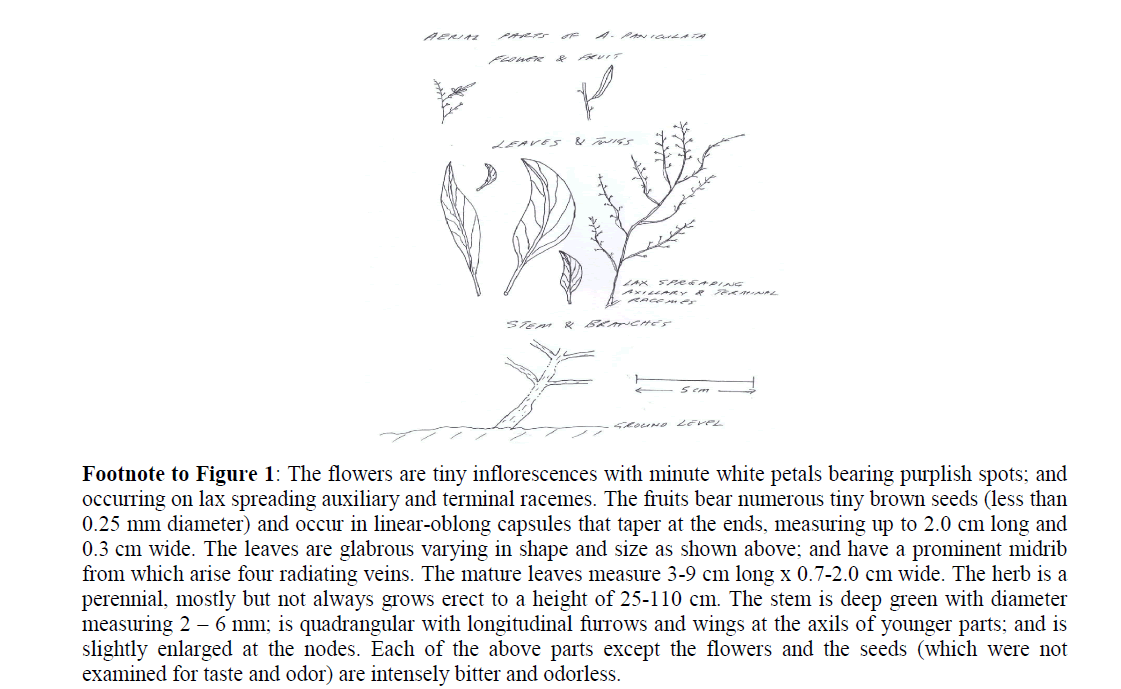
Figure 1: Aerial parts of A. paniculata with comments on habits and habitat
Footnote to Figure 1: The flowers are tiny inflorescences with minute white petals bearing purplish spots; and occurring on lax spreading auxiliary and terminal racemes. The fruits bear numerous tiny brown seeds (less than 0.25 mm diameter) and occur in linear-oblong capsules that taper at the ends, measuring up to 2.0 cm long and 0.3 cm wide. The leaves are glabrous varying in shape and size as shown above; and have a prominent midrib from which arise four radiating veins. The mature leaves measure 3-9 cm long x 0.7-2.0 cm wide. The herb is a perennial, mostly but not always grows erect to a height of 25-110 cm. The stem is deep green with diameter measuring 2 ± 6 mm; is quadrangular with longitudinal furrows and wings at the axils of younger parts; and is slightly enlarged at the nodes. Each of the above parts except the flowers and the seeds (which were not examined for taste and odor) are intensely bitter and odorless.
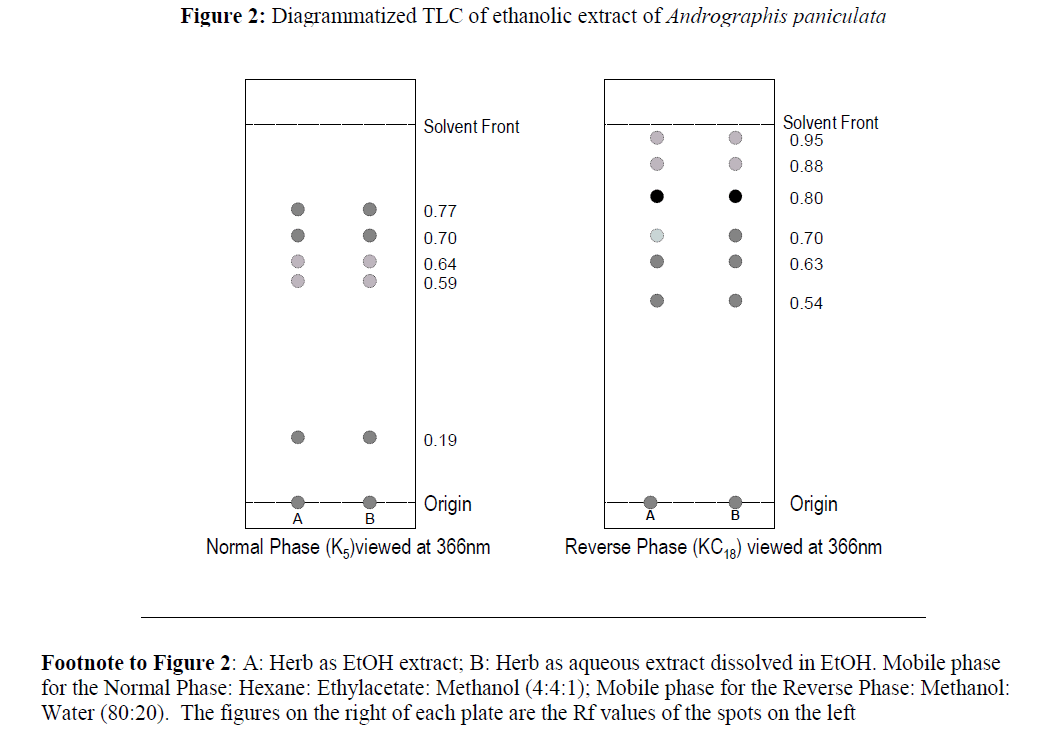
Figure 2:Diagrammatized TLC of ethanolic extract of Andrographis paniculata.
Footnote to Figure 2:A: Herb as EtOH extract; B: Herb as aqueous extract dissolved in EtOH. Mobile phase for the Normal Phase: Hexane: Ethylacetate: Methanol (4:4:1); Mobile phase for the Reverse Phase: Methanol: Water (80:20). The figures on the right of each plate are the Rf values of the spots on the left
Discussion
The gross botanical and sensory features of Andrographis paniculata are presented in Figure 1, with attention to the exceeding bitterness of its parts. Thus, even though, the most outstanding feature of herb is its bitterness, which accounts for its use as a tonic and appetite-enhancer, there is hardly any report of its determination in the literature. This could be due to a number of reasons. First, bitterness in plants is due several chemically divergent constituents that vary greatly in bitterness [6, 12, 13], hence instrumental techniques are often unsuitable for its determination. Secondly, determination of bitterness by sensation is subjective and cumbersome, and does not readily appeal to every analyst. For these reasons, the determination of bitterness by taste, as in the present instance, remains the most relevant. It is known that herbs called “bitters” are employed mostly as tonics or appetizing agents. Their bitterness stimulates secretions in the gut, especially of gastric juice [6, 12]. As indicated in Table 1, the mean bitterness value for men and women is 2.86 ± 1.74 x 103 units per g. This is about 1.43 ± 0.87 %w/w of the bitterness of quinine hydrochloride (200 x 103 units per g) [6]. The large variation is not unusual. For example Meyer and coworkers [8] had bitterness values of 58.1 ±110 x 105 and 51.6 ± 156 x 105 for Praziquantel and (R)-Praziquantel respectively. That is, Praziquantel is about 25 times as bitter as quinine. It is known that the chief bitter principle in Andrographis paniculata is andrographolide, which constitutes about 2.4 %w/w of the dry herb [5]. This may thus suggest, but not necessarily, that the bitterness of andrographolide is about 112 x 103 units/ g or 56 %w/w of the bitterness of quinine. This value is obviously a useful quantitative parameter especially in combination with the remaining results of this study. The results in Table 4 represent a major step in characterizing the Nigerian grown Andrographis. They show that the Nigerian herb compares well with the Asian variety reported by WHO [11]. Table 5 shows that the herb contains glycosides, saponins, tannins and alkaloids, but no detectable amounts of cardiac glycosides, which are toxic. Figure 2 shows that normal phase TLC of the herb or extracts yielded 5 spots as against 6 yielded by reverse TLC. Either of the chromatograms can be adopted as an identifying fingerprint for the extract. The long standing interest in Andrographis stems from the many reports of its effectiveness in the treatment of many disorders: fever, acquired immune deficiency syndrome, herpes, influenza, cancer and others [2]. Continuing interest is evident even from very recent reports of its antineoplastic properties [14] and use in treating respiratory tract infections [15]. In countries where Andrographis, processed as per good manufacturing practice (GMP) are available, they are presented as the dried herb in capsules or tablets, or as standardized extracts containing 11.2 mg of andrographolides per 200 mg of extract. For the dried herb, 0.5-3.0 g may be taken thrice daily as a tea. A typical dosage is 400 mg thrice daily [3].
Conclusion
The study confirms similarities between the Nigerian and the Asian herb; and provides valuable quantitative and descriptive data for identifying and characterizing the Nigerian grown Andrographis paniculata - an essential step in the direction of GMP-production.
Conflict of Interest: NIL
Source of support: NONE
5372
References
- Andrographis paniculata.https://www.ncbi.nlm.nih.gov/pubmed/1509 5142 .Accessed 2009 Nov 22.
- Andrographis: In-depth Review.https://www.altcancer.com/andcan.htm Accessed 2007 Sept 14.
- A-Zherbs. Andrographis paniculata (Acanthaceae) 2006. https://a-zherbs.blogspot.com/2006/11/pharmacolog y.html .Accessed 2007 Sep 26.
- Puri A, Saxena R, Saxena RP and Saxena KC. Immunostimulant agents from Andrographis paniculata. J. Natural Products 1993; 56(Pt 7):995-99.
- Sharma A, Krishan L and Handa SS. Standardization of the Indian crude drug Kalmegh by high pressure liquid chromatographic determination of andrographolide. Phytochemical analysis 1992; 3:129-31.
- Quality Control Methods for Medicinal Plant Materials. WHO, Geneva;1998. 128 p. https://aps.who.int/medicinedocs/collect/me dicinedocs/pdf/h1791e.pdf. Accessed 2008 Oct 2.
- European Pharmacopoeia, Fifth Edition, 2006. Supplement 5.7. Directorate for the Quality of Medicines of the Council of Europe. Strasbourg.
- Meyer T, Sekljic H, Fuchs S, Bothe H, Schollmeyer, D. and Miculka C. Taste, A New Incentive to Switch to (R)- Praziquantel in Schistosomiasis Treatment. PLoS Negl Trop Dis. 2009 January; 3(1): e357. Published online 2009 January 13. https://www10.1371/journal.pntd.0000357. Accessed 2010 Feb 8
- Harborne JB. Phytochemical Methods. 2nd Edition. Chapman and Hall, London and New York. ISBN 0-412-25550-2. 1984. 288 p
- Onwukaeme DN, Ikuegbvweha TB and Asonye CC. Evaluation of Phytochemical Constituents, Antibacterial Activities and Effect of Exudate of Pycanthus Angolensis Weld Warb (Myristicaceae) on Corneal Ulcers in Rabbits. Tropical J. Pharm. Res. 2007; 6(Pt 2): 725-730. https://ajol.info/index.php/tjpr/article/viewF ile/14652/2757. Accessed 2008 Dec 4
- WHO. Monograph on Andrographis paniculata. 2006. https://www.who.int/medicines/library/trm/ medicinalplants/vol2/012to024.pdf. Accessed 2006 Sep 14.
- Drewnowski A and Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. American Journal of Clinical Nutrition 2000; December 72 (Pt 6): 1424-1435.
- Drewnowski, A. 2001. The science and complexity of bitter taste. Nutr. Rev. 2001; June 59(Pt 6): 163-9.
- Varma A, Padh H, and Shrivastava N. Andrographolide: A New Plant-Derived Antineoplastic Entity on Horizon. Evidence Based Complementary Alternative Medicine. 2009 Sep 14. (Epub ahead of print) https://andrographis.thesciencenet.com/200 9/09/andrographolide-new-plant- derived.html. Accessed 2010 Feb 8.
- Saxena RC, Singh R, Kumar P, Yadav SC, Negi MP, Saxena VS, Joshua AJ, Vijayabalaji V, Goudar KS, Venkateshwarlu K, Amit A. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010 Jan 19. (Epub ahead of print)https://andrographis.thesciencenet.com/201 0/01/clinical-evaluation-of-extract-of.html . Accessed 2010 Feb 8.








