Key words
|
| |
| Cigarette smoking, Nicotine patch, Carbon nanotubes, Microneedle |
| |
INTRODUCTION
|
| |
| Transdermal drug delivery systems (TDDS), are the dosage forms, which are also known as “patches”.there designing is done in such a manner that therapeutically effective amount of drug is delivered across patient’s skin. Transdermal drug delivery has been the leading edge over injectables and oral routes by enhancing patient compliance and bypassing first pass metabolism respectively [1]. Nicotine is a tertiary amine derivative composed of a pyridine and a pyrrolidine ring. It binds to acetylcholine receptors at ganglia and neuromuscular junctions and in the brain (Benowitz, 1988). Nicotine freely permeates membranes, in its non-ionized form, including the buccal mucosa and the bloodbrain barrier. Acting as a weak base, nicotine is less ionized and penetrates membranes more easily in alkaline solutions. [2,3] |
| |
|
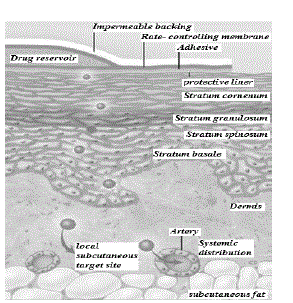
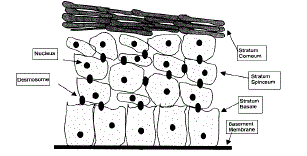
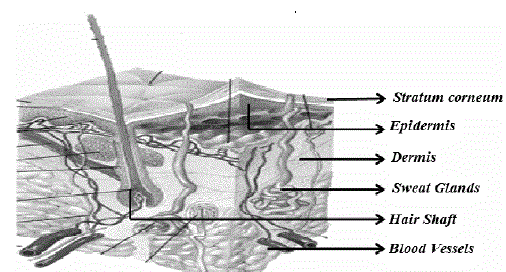
Structure of skin (Fig.1):
|
| |
| Skin is the largest organ of the integumentary system of the human body. It performs various functions like: |
| |
| 1. It keeps the body and organs intact. |
| |
| 2. It protects the internal organs from pathogens, chemicals, and other external threats. |
| |
| 3. It acts as a water resistant barrier and minimizes the trans-epidermal water loss (TEWL). |
| |
| 4. It regulates the temperature of the body by secretion and controls the energy losses by radiation, convection, and conduction. |
| |
| It acts as a storage center for water and lipids, and helps in synthesizing vitamin D. |
| |
| Skin mainly consists of three main layers: |
| |
| 1) Epidermis: It protects the body organs from infectious organisms and prevents the evaporation of water from the body. Epidermis is the outermost layer of the skin, which consists of stratified squamous epithelium with an underlying basal lamina. It can be further divided into five distinct layers, which provides barrier resistance to the skin. The five layers of the epidermis are arranged in the order from top to bottom are stratum corneum, stratum lucidum (In palms of hands and bottoms of feet), stratum granulosum, stratum spinosum and stratum basale. [5] |
| |
| 2) Dermis: It comprises of connective tissues and cushions the body from stress and strain. It is a 2-3 mm thick layer present beneath the epidermis and provides flexible support structure to the skin. It has hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels including blood vessels. This layer provides the synthesis of collagen and elastin. Dermis is tightly linked to the epidermis by a basement membrane and helps in regulation of temperature, pain and pressure in the body. The sense of touch and heat to the skin are provided by the nerve endings that are present in this layer. |
| |
| 3) Hypodermis: It consists of subcutaneous adipose tissues and mainly acts as a thermal barrier. It is the deepest layer of the skin, also known as subcutaneous tissue. It includes adipose tissue (fat cells), connective tissues, macrophages and fibroblasts. [6] |
| |
|
Pathogenic effects of Nicotine:
|
| |
| About 4,000 or more chemical entities of tobacco smoke, along with the huge arsenal of toxins and carcinogens, act as the mediators of multiple pathogenic processes. Nicotine, a tertiary amine, should by no means be considered an inert molecule. For example, nicotine can cause vasoconstriction in specific vascular beds but dilatation in others. Nicotine can raise the heart rate (by 10–15 beats/min) and the blood pressure (by 5– 10 mm Hg) and can induce pathogenic changes to the endothelium that are associated with the atherosclerotic mechanism. Although smoking is implicated in the development of cancer, nicotine itself is not carcinogenic, unless it undergoes nitrosation to form nitrosamines (a mechanism known to occur during tobacco curing and combustion). The need remains for further studies on the pathogenic potential of nicotine. [9-13] |
| |
|
Components of a transdermal patch:
|
| |
| Transdermal patch may consist of the following components: |
| |
| 1. Liner -It protects the patch during storage. It is removed prior to use. |
| |
| 2. Drug - Drug solution is in direct contact with release liner. |
| |
| 3. Adhesive -It serve to adhere the components of the patch together along with adhering the patch to the skin. |
| |
| 4. Membrane -It is used to control the release of the drug from the reservoir and multi-layer patches. |
| |
| 5. Backing -It protects the patch from the outer environment. [14-16] |
| |
|
Types of transdermal patches
|
| |
| There are mainly five types of transdermal patches listed below: |
| |
| 1. Single-layer Drug-in-Adhesive: The adhesive layer of this system is also provided with the drug. The adhesive layer is responsible for adhering the various layers together, along with the release of drug. The adhesive layer is further surrounded by a temporary liner and a backing. |
| |
| 2. Multi-layer Drug-in-Adhesive: The multilayer drug-in adhesive patch has a close similarity with single-layer system in that ,both adhesive layers are responsible for the release of the drug. The multi-layer system has additional layer of drug-in-adhesive, that is usually separated by a membrane (in many cases). This type of patch also has a permanent backing associated with the temporary liner layer. |
| |
| 3. Drug Reservoir-In-Adhesive: Unlike the Single-layer and Multi-layer Drug-in adhesive systems the drug reservoir–in-adhesive system has a separate drug layer. The drug layer is in the form of drug solution or suspension that has been separated by the adhesive layer. Backing layer is also provided with this patch. Zero order kinetics is followed in this type of system. |
| |
| 4. Drug Matrix-In-Adhesive: In this type of transdermal patch, the Matrix system is associated with a drug layer which is in the form of semisolid matrix containing a drug solution or suspension. The adhesive layer in this patch surrounds the drug layer partially overlaying it. |
| |
| 5. Vapour Patch: The vapour patch performs basically two functions, first one is to adhere the various layers and the second one is to release vapour. The vapour patches have been introduced recently in the market and essential oils are released from them for up to 6 hours, due to this property,the vapour patches are used in cases of decongestion mainly. Controller vapour patches are also present in the market that improve the quality of sleep. In the market there is also availability of Vapour patches that have been required to reduce the quantity of cigarettes . [17-19] |
| |
|
Use of transdermal patch
|
| |
| Transdermal patches are used when: |
| |
| 1. When side effects are intolerable (including constipation) in the patient and who is finding difficulty in oral medication (dysphagia). |
| |
| 2. Where the pain control might be improved by suitable administration. This might be useful in patients suffering from cognitive impairment or those who for other reasons are not able to self-medicate with their analgesia. |
| |
| 3. It produces synergistic effects when used in combination with other enhancement strategies. [20] |
| |
|
Unfavorable condition for transdermal patch used:
|
| |
| 1. When there is acute pain condition. |
| |
| 2. Where rapid dose titration is required. |
| |
| 3. Where requirement of dose is equal to or less then 30 mg/24 hrs. |
| |
|
Care taken while using transdermal patch:
|
| |
| 1. The part of the skin should be properly cleaned where the patch is to be applied. |
| |
| 2. The system of patch should be free from cut because the drug delivery system is destroyed by the cut in the patch. |
| |
| 3. Before applying a new patch it should me made clear enough that the old patch is removed from the site. |
| |
| 4. Patch should be applied and removed carefully because the persons handling the patch can absorb the drug from the patch. |
| |
| 5. There should be accurate application of patch to the site of administration |
| |
|
Factors influencing transdermal delivery:
|
| |
| 1. Medicament release from the vehicle. |
| |
| 2. The extent of penetration through the skin barrier. |
| |
| 3. Pharmacological response activation. [21] |
| |
|
Recent advancements in transdermal drug delivery:
|
| |
| 1) Carbon Nanotube Membranes: Recently, a novel skin patch tool for the delivery of the nicotine was developed by Dr. Hinds and his colleagues which were basically based on the technology in which Carbon nanotubes (CNT) of approximate diameter 2- 6.5 nm are aligned in such a way to form an active layer in a solid film of polymer. During the process both ends Carbon nanotubes are adapted in such a way that by mere applying and removing the electric current the cross-linking can be opened or closed. |
| |
| In his research, Dr. Hinds had shown that when the carbon nanotubes are in the patch are arranged in the open condition, molecules having small molecular weight can be pumped through the patch atleast five times quicker. So, the CNT patch can also be considered as system for the delivery of the drug or nicotine that can be controlled by the physician or the user in order to obtain the comparable plasma levels of nicotine as associated with the cigarette smoking and then allowed to return to the normal levels slowly by closing the CNT ends by removing the electric current. Besides the controlled transdermal delivery of the Nicotine, the CNT patch can also be used for other skin absorbable drugs. [22] |
| |
| 2) Microneedle-Based Transdermal Drug Delivery System: Transdermal drug delivery involves the passing of pharmaceutical compound across the dermis of the skin for subsequent systemic distribution. Strictly, this does not only include the more commonly understood “patch”, but also most common subcutaneous administration by means of a hypodermic needle and a syringe. Common to all methods of transdermal drug delivery, in its broader definition, is that there is passing of drug through an artificial route into the body. The most important advantage of this approach is that the drug is released into the body undistorted without passing the body’s various defense systems. In contrast to oral administration, the most easiest way of drug administration, the transdermal route does not suffer from drug degradation in the gastrointestinal tract and decreased potency through first-pass metabolism (i.e. in the liver). Further, the oral-specific sideeffects, such as liver damages, are avoided, which are common with the drugs like estradiol (estrogen) [23] or paracetamol. [24] |
| |
|
Permeation enhancers mechanical methods:
|
| |
| The various classes of active systems under development include iontophoresis, electroporation, micro needles, Abrasion, needle-less injection, suction, stretching, ultrasound, magnetophoresis, radio frequency, lasers, photomechanical waves, and temperature manipulation. |
| |
| Some most commonly employed techniques include the following. |
| |
| 1. Iontophoresis: In this method, low level electric current are applied either directly to the skin or indirectly via suitable dosage form in order to enhance the permeation efficiency of a topically applied therapeutic agent.[25-28] Increased drug permeation through this methodology can be attributed to either one or a combination of the following mechanisms: Electro-repulsion (for charged solutes), electro-osmosis (for uncharged solutes) and electro-pertubation (for both charged and uncharged). |
| |
| 2. Electroporation: In this method, the application of high voltage pulses to the skin that has been suggested to induce the formation of transient pores. In this process, there is frequent application of high voltages (100 V) and short treatment durations (milliseconds). Electroporation has been successfully employed in the enhancement of the skin permeability of molecules that are having differing lipophilicity and size (i.e. small molecules, proteins etc.) along with the biopharmaceuticals (molecular weights greater than 7kDA). |
| |
| 3. Microneedle-Based Devices: The first microneedle based system was, described in 1976, which consist of a drug reservoir and a plurality of projections (micro needles 50 to 100 mm long) and extends from the reservoir, which penetrated the stratum corneum and epidermis for delivering the drug moiety. [29] |
| |
| 4. Skin Abrasion: In this technique, the upper layers of the skin is directly removed or disrupted, so that it easily helps in permeation of topically applied medicaments. There are also some devices that are based on techniques which are employed by dermatologists for superficial skin resurfacing (e.g. microdermabrasion) that have use in the treatment of acne, scars, hyper pigmentation and other skin blemishes. |
| |
| 5. Needle-Less Injection: In this transdermal delivery system, the liquid or solid particles are fired at supersonic speeds through the outer layers of the skin using a reliable energy source for delivering the drug. The mechanism is basically, forcing compressed gas (helium) via a nozzle, such that the resultant drug particles entrained within the jet flow that travels at sufficient velocity for skin penetration. |
| |
| 6. Ultrasound (Sonophoresis and Phonophoresis): In this technique, there is requirement of ultrasonic energy, to increase the transdermal delivery of solutes. Low frequency ultrasound (55 kHz) is required for an time duration of 15 sec, leads to the enhancement of the skin’s permeability. [30] |
| |
| 7. Laser Radiation: In this technique, skin is exposed to direct and controlled laser beam that results in the ablation of the stratum corneum without significantly affecting the underlying epidermis. Stratum corneum has been removed efficiently by this method that has led to the enhancement of the delivery of lipophilic and hydrophilic drugs. [31] |
| |
|
Advantages of transdermal drug delivery:
|
| |
| 1. Reduction of dosing frequency, due to longer duration of action. |
| |
| 2. It is convenient in administration of the drugs. |
| |
| 3. It has lead to improved bioavailability. |
| |
| 4. It provides more uniform plasma levels. |
| |
| 5. Side effects have been reduced significantly and improved therapy due to maintenance of plasma levels up to the end of the dosing interval. |
| |
| 6. Drug administration can be easily terminated by simply removing the patch from the skin. |
| |
| 7. It has lead to the improved patient compliance and comfort through non-invasive, painless and simple application. |
| |
DISADVANTAGES OF TRANSDERMAL DRUG DELIVERY:
|
| |
| 1. There is possibility of local irritation at the site of application. |
| |
| 2. There may be problem of Erythema, itching, and local edema due to the drug, the adhesive, or other excipients in the formulation of a patch .[32] |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |









