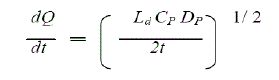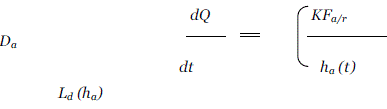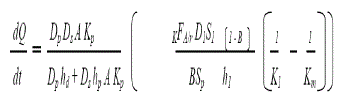Key words
|
| |
| Transdermal, Drug Delivery, TDDS, Novel drug delivery system. |
| |
Introduction
|
| |
| Optimum therapeutic outcomes require not only proper drug selection but also effective drug delivery. The human skin is a readily accessible surface for drug delivery. Over the past three decades developing controlled drug delivery has become increasingly important in the pharmaceutical industry. The pharmacological response, both the desired therapeutic effect and the undesired adverse effect, of a drug is dependent on the concentration of the drug at the site of action, which in turn depends upon the dosage form and the extent of absorption of the drug at the site of action1. Tablets and injections have been the traditional way to take medications; new options are becoming increasingly popular. One highly successful alternative delivery method is the transdermal. Skin of an average adult body covers a surface of approximately 2 m2 and receives about one-third of the blood circulating through the body. The deliver a drug into the body through transdermal layer of skin, it is necessary to understand about the skin. |
| |
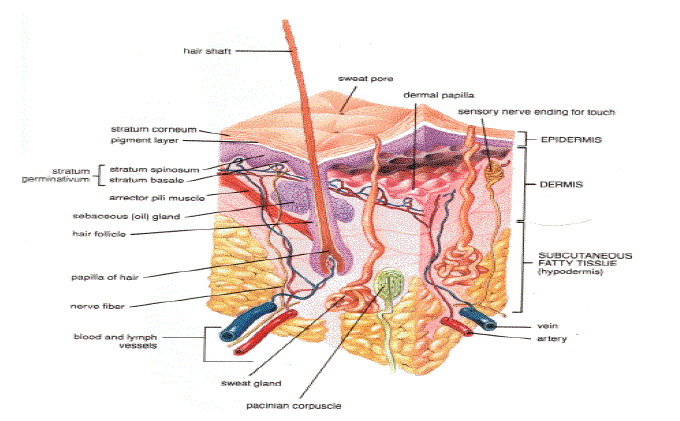
| Anatomy and physiology of skin: Human skin comprises of three distinct but mutually dependent tissues 2-5: |
| |
| A) The stratified, vascular, cellular epidermis, |
| |
| B) Underlying dermis of connective tissues and |
| |
| C) Hypodermis. |
| |
|
Epidermis
|
| |
| The multilayered epidermis varies in thickness, depending on cell size and number of cell layers of epidermis, ranging from 0.8 mm on palms and soles down to 0.06 mm on the eyelids. Table 1 gives thickness, water permeability and diffusivity of water through epidermis. It consists outer stratum corneum and viable epidermis. |
| |
|
a) Stratum corneum
|
| |
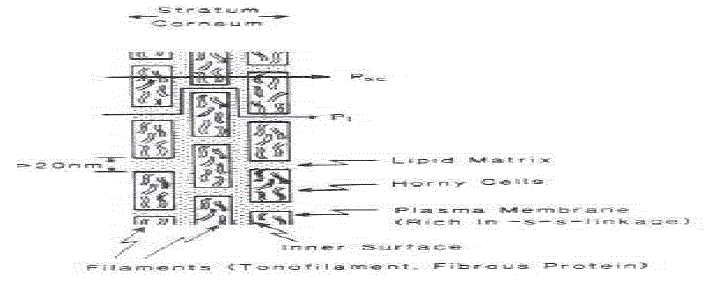
| This is the outermost layer of skin also called as horney layer. It is approximately 10mm thick when dry but swells to several times this thickness when fully hydrated. It contains 10 to 25 layers of dead, keratinized cells called corneocytes. It is flexible but relatively impermeable. The stratum corneum is the principal barrier for penetration of drug. The architecture of horney layer may be modeled as a wall-like structure. In this model, the keratinized cells function as protein “bricks” embedded in lipid “mortar.” The lipids are arranged in multiple bilayers. There is sufficient amphiphilic material in the lipid fraction, such as polar free fatty acids and cholesterol, to maintain a bilayer form. |
| |
|
b) Viable epidermis
|
| |
| This is situated beneath the stratum corneum and varies in thickness from 0.06mm on the eyelids to 0.8mm on the palms. Going inwards, it consists of various layers as stratum lucidum, stratum granulosum, stratum spinosum and the stratum basal. In the basale layer, mitosis of the cells constantly renews the epidermis and this proliferation compensates the loss of dead horney cells from the skin surface. As the cells produced by the basal layer move outward, they alter morphologically and histochemically, undergoing keratinization to form the outermost layer of stratum corneum. |
| |
|
Dermis
|
| |
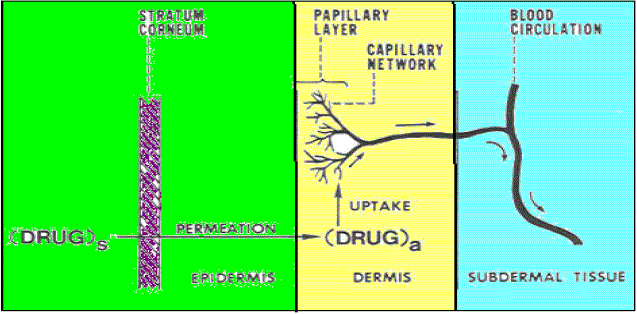
| Dermis is 3 to 5mm thick layer and is composed of a matrix of connective tissue, which contains blood vessels, lymph vessels and nerves. The cutaneous blood supply has essential function in regulation of body temperature. It also provides nutrients and oxygen to the skin while removing toxins and waste products. Capillaries reach to within 0.2 mm of skin surface and provide sink conditions for most molecules penetrating the skin barrier. The blood supply thus keeps the dermal concentration of a permeant very low and the resulting concentration difference across the epidermis provides the essential concentration gradient for transdermal permeation. |
| |
|
Hypodermis
|
| |
| The hypodermis or subcutaneous fat tissue supports the dermis and epidermis. It serves as a fat storage area. This layer helps to regulate temperature, provides nutritional support and mechanic al protection. It carries principal blood vessels and nerves to skin and may contain sensory pressure organs. |
| |
| For transdermal drug delivery, drug has to penetrate through all these three layers and reach into systemic circulation while in case of topical drug delivery only penetration through stratum corneum is essential and then retention of drug in skin layers is desired. |
| |
|
Fundamentals of skin permeation 6
|
| |
| Until the last century the skin was supposed to be impermeable with exception to gases. However, in the current century the study indicated the permeability to lipid soluble drugs. Also it was recognized that various layers of skin are not equally permeable i.e. epidermis is less permeable than dermis. After a large controversy, all doubts about stratum corneum permeability were removed and using isotopic tracers, it was suggested that stratum corneum greatly hamper permeation. |
| |
|
A. Stratum corneum as skin permeation barrier
|
| |
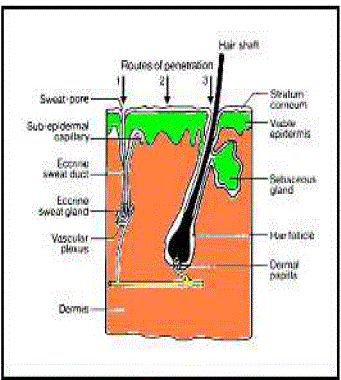
| The average human skin contains 40-70 hair follicles and 200-250 sweat ducts per square centimeter. Especially water-soluble substances pass faster through these ducts, still these ducts don’t contribute much for skin permeation. Therefore most neutral molecules pass through stratum corneum by passive diffusion. Regional variation in water permeability of stratum corneum showed in Table 1 and permeation of drug molecule through skin showed in Figure 2. |
| |
| Series of steps in sequence: |
| |
| 1. Sorption of a penetrant molecule on surface layer of stratum corneum. |
| |
| 2. Diffusion through it and viable epidermis and finally reaches to dermis and then |
| |
| 3. The molecule is taken up into the microcirculation for systemic distribution. |
| |
|
B-Intracellular verses transcellular diffusion
|
| |
| Intracellular regions in stratum corneum are filled with lipid rich amorphous material. In dry stratum corneum intracellular volume may be 5% to 1% in fully hydrated stratum corneum |
| |
|
C. Permeation pathways 7-9
|
| |
| Percutaneous absorption involves passive diffusion of the substances through the skin. A molecule may use two diffusional routes to penetrate normal intact skin, the appendageal route and the epidermal route. |
| |
|
1. Appendageal route:
|
| |
| Appendageal route comprises transport via sweat glands and hair follicles with their associated sebaceous glands. These routes circumvent penetration through the stratum corneum and are therefore known as “shunt” routes. This route is considered to be of minor importance because of its relatively small area, approximately 0.1 % of the total skin area. |
| |
|
1. Epidermal route:
|
| |
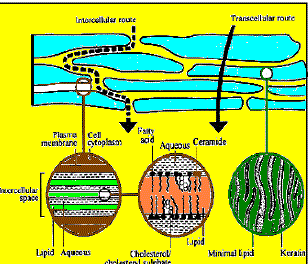
| For drugs, which mainly cross-intact horney layer, two potential micro routes of entry exists, the transcellular (intracellular) and intercellular pathways. |
| |
| i)Transcellular: Transcellular pathway means transport of molecules across epithelial cellular membrane. These include passive transport of small molecules, active transport of ionic and polar compounds and endocytosis and transcytosis of macromolecules. |
| |
| ii) Paracellular: Paracellular pathway means transport of molecules around or between the cells. Tight junctions or similar situations exist between the cells. |
| |
| The principal pathway taken by a permeant is decided mainly by the partition coefficient (log k). Hydrophilic drugs partition preferentially into the intracellular domains, whereas lipophilic permeants traverse the stratum corneum via the intercellular route. Most permeants permeate the stratum corneum by both routes. However, the tortuous intercellular pathway is widely considered to provide the principal route and major barrier to the permeation of most drugs. |
| |
|
Properties that influence transdermal drug delivery 10-11
|
| |
| The effective transdermal drug delivery can be formulated by considering three factors as drug, skin and the vehicles. So the factors affecting can be divided in two classes as biological factors and physicochemical factors. |
| |
|
A. Biological factors
|
| |
| i) Skin condition: Acids and alkalis, many solvents like chloroform, methanol damage the skin cells and promote penetration. Diseased state of patient alters the skin conditions. The intact skin is better barrier but the above mentioned conditions affect penetration. |
| |
| ii) Skin age: The young skin is more permeable than older. Childrens are more sensitive for skin absorption of toxins. Thus, skin age is one of the factor affecting penetration of drug in TDDS. |
| |
| iii) Blood supply: Changes in peripheral circulation can affect transdermal absorption. |
| |
| iv) Regional skin site: Thickness of skin, nature of stratum corneum and density of appendages vary site to site. These factors affect significantly penetration. |
| |
| v) Skin metabolism: Skin metabolizes steroids, hormones, chemical carcinogens and some drugs. So skin metabolism determines efficacy of drug permeated through the skin. |
| |
| vi) Species differences: The skin thickness, density of appendages and keratinization of skin vary species to species, so affects the penetration. |
| |
|
B. Physicochemical factors
|
| |
| i) Skin hydration: In contact with water the permeability of skin increases significantly. Hydration is most important factor increasing the permeation of skin. So use of humectant is done in transdermal delivery. |
| |
| ii) Temperature and pH: The permeation of drug increase ten folds with temperature variation. The diffusion coefficient decreases as temperature falls. Weak acids and weak bases dissociate depending on the pH and pKa or pKb values. The proportion of unionized drug determines the drug concentration in skin. Thus, temperature and pH are important factors affecting drug penetration. |
| |
| iii) Diffusion coefficient: Penetration of drug depends on diffusion coefficient of drug. At a constant temperature the diffusion coefficient of drug depends on properties of drug, diffusion medium and interaction between them. |
| |
| iv) Drug concentration: The flux is proportional to the concentration gradient across the barrier and concentration gradient will be higher if the concentration of drug will be more across the barrier. |
| |
| v) Partition coefficient: The optimal partition coefficient (K) is required for good action. Drugs with high K are not ready to leave the lipid portion of skin. Also, drugs with low K will not be permeated. |
| |
| vi) Molecular size and shape: Drug absorption is inversely related to molecular weight, small molecules penetrate faster than large ones. |
| |
|
Ideal molecular properties for transdermal drug delivery 12
|
| |
| From the above considerations we can conclude with some observations that can termed as ideal molecular properties for drug penetration. They are as follows. |
| |
| • An adequate solubility in lipid and water is necessary for better penetration of drug (1mg/ml). |
| |
| • Optimum partition coefficient is required for good therapeutic action. |
| |
| •Low melting point of drug is desired (<200°C). |
| |
| •The pH of the saturated solution should be in between 5 to 9. |
| |
|
Design of transdermal delivery system 10
|
| |
| The basic components of any transdermal delivery system include the drug dissolved or dispersed in an inert polymer matrix that provides support and platform for drug release. There are two basic designs of the patch system that dictate drug release characteristics and patch behavior: |
| |
| i) Matrix or Monolithic: The inert polymer matrix binds with the drug and controls it’s release from the device. |
| |
| ii) Reservoir or Membrane: The polymer matrix does not control drug release. Instead, a ratecontrolling membrane present between the drug matrix and the adhesive layer provides the ratelimiting barrier for drug release from the device. |
| |
|
Technologies for developing transdermal drug delivery systems 4, 6, 11
|
| |
| The technologies can be classified in four basic approaches: |
| |
|
A) Polymer membrane partition-controlled TDD systems
|
| |
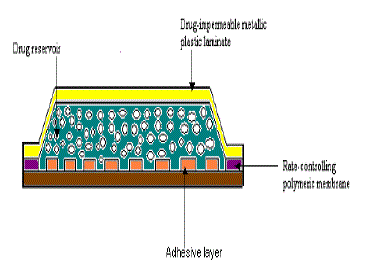
| In this type of systems, the drug reservoir is sandwiched between a drug-impermeable backing laminate and a rate controlling polymeric membrane. |
| |
| The drug is allowed to permeate only through the rate controlling membrane. The drug solids are homogeneously dispersed in a solid polymer matrix, suspended in an unleachable, viscous liquid medium e.g. silicone fluid to form a paste like suspension or dissolved in a releasable solvent e.g. alkyl alcohol to form a clear drug solution. The rate controlling membrane can be either a microporous or a nonporous polymeric membrane e.g. ethylene-vinyl acetate copolymer, with specific drug permeability. On the external surface of the polymeric membrane a thin layer of drug-compatible, hypoallergenic pressure sensitive adhesive polymer e.g. silicone adhesive may be applied to provide intimate contact of TDDS with the skin surface. |
| |
| Varying the composition of drug reservoir formulation, the permeability coefficient and thickness of rate controlling membrane can alter the drug release rate. |
| |
| e.g. Some FDA approved systems – Transderm- Nitro for angina pectoris, Transderm-Scop for motion sickness, Catapres-TTS system for hypertension. |
| |
| The intrinsic rate of drug release from this type of TDD system is defined by |
| |
 |
| |
| where, CR is drug concentration in reservoir compartment. |
| |
| Km / rthe partition coefficient for the interfacial partitioning of drug from the reservoir to the membrane. |
| |
| Ka / m the partition coefficient for the interfacial partitioning of drug from membrane to adhesive. |
| |
| Da diffusion coefficient in rate controlling membrane. |
| |
| Dm diffusion coefficient in adhesive layer. |
| |
| ha thickness of rate controlling membrane. |
| |
| hm thickness of adhesive layer. |
| |
|
B) Polymer matrix diffusion-controlled TDD systems
|
| |
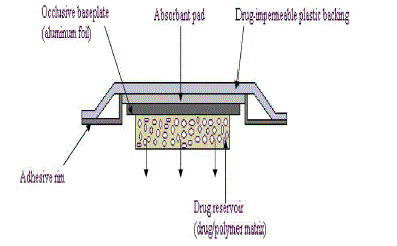
| In this system, the drug reservoir is formed by homogeneously dispersing the drug solids in a hydrophilic or lipophilic polymer matrix and then the medicated polymer formed is molded into medicated disks with defined surface area and thickness. This drug reservoir containing polymer disk is then mounted on occlusive baseplate in a compartment fabricated from a drug-impermeable plastic backing. |
| |
| Instead of coating adhesive polymer directly on the surface of medicated disk, it is applied along the circumference of the patch to form a strip of adhesive rim surrounding the medicated disk. e.g. Nitro-Dur system and NTS system for angina pectoris. |
| |
| The rate of release from polymer matrix drug dispersion-type is |
| |
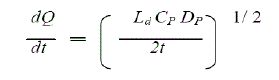 |
| |
| where, Ldis drug loading dose initially dispersed in polymer matrix. |
| |
| CP is solubility of drug in polymer matrix. |
| |
| DP is diffusivity of drug in polymer matrix. |
| |
| Only drug dissolved in polymer matrix can diffuse, CP is practically equal to CR |
| |
| Alternately, the polymer matrix drug dispersiontype TDDS can be fabricated by directly dispersing drug in a pressure-sensitive adhesive polymer e.g. polyacrylate and then coating the drug-dispersed adhesive polymer by solvent casting or hot melt onto a flat sheet of drug-impermeable backing laminate to form a single layer of drug reservoir, this yields a thinner patch.eg. Minitran system, Nitro-Dur II system for angina pectoris. |
| |
|
C) Drug reservoir gradient-controlled TDD systems
|
| |
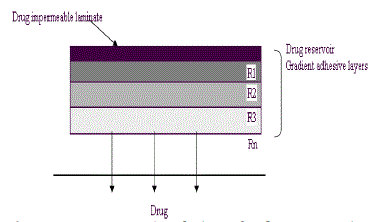
| Polymer matrix drug dispersion-type TDDS can be modified to have the drug loading level varied in an incremental manner, forming a gradient of drug reservoir along the diffusional path across the multilaminate adhesive layers. The drug release from this type of drug reservoir gradient- controlled TDDS can be expressed by |
| |
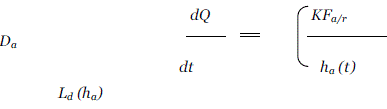 |
| |
| In this system, the thickness of diffusional path through which drug molecules diffuse increases with time i.e. ha (t). The drug loading level in the multilaminate adhesive layer is designed to increase proportionally i.e. Ld (ha) so as to compensate time dependent increase in diffusional path as a result of drug depletion due to release. Thus, theoretically this should increase a more constant drug release profile. eg. Deponit system containing nitroglycerine for angina pectoris. |
| |
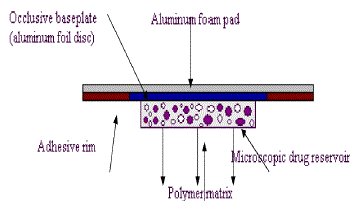
| D. Microreservoir dissolution-controlled TDD systems |
| |
| A hybrid of reservoir and matrix dispersion-type drug delivery systems, which contains dug reservoir formed by first suspending the drug solids in an aqueous solution of water-miscible drug solubilizer e.g. propylene glycol, then homogeneously dispersing the drug suspension with controlled aqueous solubility in a lipophilic polymer by high shear mechanical force to form thousands of unleachable microscopic drug reservoirs. |
| |
|
Microreservoir dissolution-controlled TDD systems
|
| |
| This thermodynamically unstable system is quickly stabilized by immediately cross-linking the polymer chains in situ, which produces a medicated polymer disk with a constant surface area and a fixed thickness. Medicated disk is mounted at the center of an adhesive pad. |
| |
| eg. Nitrodisk system for angina pectoris. |
| |
| The rate of drug release from this system is defined by : |
| |
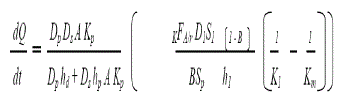 |
| |
|
Components of TDDS 13
|
| |
| The main components of a transdermal patch are: |
| |
|
i. Release Liner
|
| |
| Protects the patch during storage. The liner is removed prior to use. |
| |
|
ii. Drug reservoir
|
| |
| The most important part of TDDS is drug reservoir. It consists of drug particles dissolved or dispersed in the matrix. To make the drug soluble, solvents and cosolvents are used. The effect of solvent and cosolvent should be considered while doing selection. |
| |
|
iii. Adhesive
|
| |
| Serves to adhere the components of the patch together along with adhering the patch to the skin. The adhesive must posses sufficient adhesion property so that the TDDS should remain in place for a long time. Pressure sensitive adhesives are commonly used for transdermal patch to hold the skin. Commonly used adhesives are silicone adhesives, poly iso butylenes adhesives and poly acrylate based adhesives. |
| |
|
iv. Membrane
|
| |
| Membrane controls the release of the drug from the reservoir and multi-layer patches. It may or may not contain rate-controlling membrane. It should be flexible enough not to split or crack on bending or stretching. Some of rate-controlling membranes are polyethylene sheets, ethylene vinyl acetate copolymer and cellulose acetate. |
| |
|
v. Backing
|
| |
| Protects the patch from the outer environment. The backing layer should be impermeable to drug and penetration enhancers. It serves a function of holding the entire system and protects drug reservoir from atmosphere. The commonly used backing materials are polyesters, aluminized polyethylene terepthalate and siliconized polyethylene terepthalate. |
| |
|
General clinical considerations in the use of TDDS 7, 13
|
| |
| The patient should be advised of the following general guidelines. The patient should be advised of the importance of using the recommended site and rotating locations within the site. Rotating location is important to allow the skin to regain its normal permeability and to prevent skin irritation. |
| |
| 1. TDDS should be applied to clean, dry skin relatively free of hair and not oily, inflamed, irritated, broken. Wet or moist skin can accelerate drug permeation time. Oily skin can impair the adhesion of patch. If hair is present at the site, it should be carefully cut, not wet shaved nor should a depilatory agent be used, since later can remove stratum corneum and affect the rate and extent of drug permeation. |
| |
| 2. Use of skin lotion should be avoided at the application site, because lotions affect the hydration of skin and can alter partition coefficient of drug. |
| |
| 3. Patient should not physically alter TDDS, since this destroys integrity of the system. |
| |
| 4. The protecting backing should be removed with care not to touch fingertips. The TDDS should be pressed firmly against skin site with the heel of hand for about 10 seconds. |
| |
| 5. A TDDS should be placed at a site that will not subject it to being rubbed off by clothing or movement. TDDS should be left on when showering, bathing or swimming. |
| |
| 6. A TDDS should be worn for full period as stated in the product’s instructions followed by removal and replacement with fresh system. |
| |
| 7. The patient or caregiver should clean the hands after applying a TDDS. Patient should not rub eye or touch the mouth during handling of the system. |
| |
| 8. If the patient exhibits sensitivity or intolerance to a TDDS or if undue skin irritation results, the patient should seek reevaluation. |
| |
| 9. Upon removal, a used TDDS should be folded in its half with the adhesive layer together so that it cannot be reused. The used patch discarded in a manner safe to children and pets. |
| |
| It is important to use a different application site everyday to avoid skin irritation. |
| |
| Suggested rotation is : |
| |
| Day 1 – Upper right arm |
| |
| Day 2 – upper right chest |
| |
| Day 3 – Upper left chest |
| |
| Day 4 – Upper left arm, |
| |
| then repeat from Day 1. |
| |
|
Transdermal patches 9
|
| |
| Both topical and transdermal products are intended for external application. However, topical dermatological products are intended for local action whereas transdermal drug delivery system is used for systemic drug delivery. |
| |
| Transdermal system delivers medications through the skin direct into the bloodstream. The transdermal route of drug delivery is becoming popular because large number of drugs can be delivered by this route to treat various diseases. Currently, transdermal patches are used in several therapeutic areas like pain management, smoking cessation, treatment of heart disease, hormone replacement and management of motion sickness. |
| |
TYPES OF TRANSDERMAL PATCHES: 18-22
|
| |
|
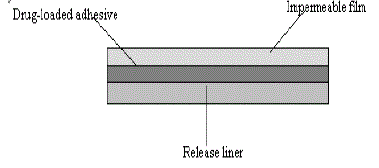
a) Single layer drug in adhesive:
|
| |
| In this type the adhesive layer contains the drug. The adhesive layer not only serves to adhere the various layers together and also responsible for the releasing the drug to the skin. The adhesive layer is surrounded by a temporary liner and a backing. |
| |
|
b) Multi -layer drug in adhesive:
|
| |
| This type is also similar to the single layer but it contains a immediate drug release layer and other layer will be a controlled release along with the adhesive layer. The adhesive layer is responsible for the releasing of the drug. This patch also has a temporary liner-layer and a permanent backing. |
| |
|
c) Vapour patch:
|
| |
| In this type of patch the role of adhesive layer not only serves to adhere the various layers together but also serves market, commonly used for releasing of essential oils in decongestion. Various other types of vapor patches are also available in the market which are used to improve the quality of sleep and reduces the cigarette smoking conditions. |
| |
|
d) Reservoir system:
|
| |
| In this system the drug reservoir is embedded between an impervious backing layer and a rate controlling membrane. The drug releases only through the ratecontrolling membrane, which can be micro porous or non porous. In the drug reservoir compartment, the drug can be in the form of a solution, suspension, gel or dispersed in a solid polymer matrix. Hypoallergenic adhesive polymer can be applied as outer surface polymeric membrane which is compatible with drug. |
| |
|
e) Matrix system:
|
| |
|
i. Drug-in-adhesive system:
|
| |
| In this type the drug reservoir is formed by dispersing the drug in an adhesive polymer and then spreading the medicated adhesive polymer by solvent casting or melting (in the case of hot-melt adhesives) on an impervious backing layer. On top of the reservoir, unmediated adhesive polymer layers are applied for protection purpose. |
| |
|
ii. Matrix-dispersion system:
|
| |
| In this type the drug is dispersed homogenously in a hydrophilic or lipophilic polymer matrix. This drug containing polymer disk is fixed on to an occlusive base plate in a compartment fabricated from a drug impermeable backing layer. Instead of applying the adhesive on the face of the drug reservoir, it is spread along with the circumference to form a strip of adhesive rim. |
| |
|
f) Microreservoir system:
|
| |
| In this type the drug delivery system is a combination of reservoir and matrix-dispersion system. The drug reservoir is formed by first suspending the drug in an aqueous solution of water soluble polymer and then dispersing the solution homogeneously in a lipophilic polymer to form thousands of unreachable, microscopic spheres of drug reservoirs. This thermodynamically unstable dispersion is stabilized quickly by immediately cross-linking the polymer in situ by using cross linking agents. |
| |
|
Advances in transdermal patch technology
|
| |
| i) Adhesives: For transdermal patches to provide consistent and continuous drug delivery through the skin, they must adhere well. There are several adhesives in regular use in transdermal systems including silicones and polyisobutylenes. Newer adhesives, including acrylates, have recently become available. Acrylates are known as pressure-sensitive adhesives and they are preferred because they have selective adhesive properties once in contact with the skin. Pressure-sensitive adhesives can also be removed with ease and they are compatible with skin and various drug molecules. The polymeric nature of acrylates prevents the patches from undergoing physical breakdown. |
| |
| i) Penetration Enhancers: Many drug substances will not diffuse into the skin at sufficient rates to obtain therapeutic concentrations Substances that reduce the skin’s ability to perform its barrier function are collectively known as penetration enhancers. These substances make the skin more permeable and they allow drug molecules to cross the skin at a faster rate. With regard to both safety and efficacy, water is the optimum permeation enhancer. By increasing the hydration of the stratum corneum, the barrier function of the skin can be reduced. Alcohol is commonly considered a solvent in transdermal patches; it actually serves as effective penetration enhancer. Some penetration enhancers remove lipids from the skin.23-25 |
| |
EVALUATION PARAMETERS: 26
|
| |
| 1. Interaction studies |
| |
| 2. Thickness of the patch |
| |
| 3. Weight uniformity |
| |
| 4. Folding endurance |
| |
| 5. Percentage Moisture content |
| |
| 6. Percentage Moisture uptake |
| |
| 7. Water vapour permeability (WVP) evaluation |
| |
| 8. Drug content |
| |
| 9. Uniformity of dosage unit test |
| |
| 10. Polariscope examination |
| |
| 11. Shear Adhesion test |
| |
| 12. Peel Adhesion test |
| |
| 13. Thumb tack test |
| |
| 14. Flatness test |
| |
| 15. Percentage Elongation break test |
| |
| 16. Rolling ball tack test |
| |
| 17. Quick Stick (peel-tack) test |
| |
| 18. Probe Tack test |
| |
| 19. In vitro drug release studies |
| |
| 20. In vitro skin permeation studies |
| |
| 21. Skin Irritation study |
| |
| 22. Stability studies |
| |
|
Animal Models to Study Transdermal Absorption 15-17
|
| |
| Ex vivo penetration and permeation are routinely performed to study percutaneous absorption and transdermal permeation characteristics of drugs and other chemicals. These ex vivo studies allow the determination of drug concentration in the skin (penetration) and rate of transfer across the skin (permeation). Ex vivo experiments are easy to perform and the simplicity of methodology allows flexibility in adapting the model in addressing different aspects involved in preliminary or feasibility studies in the development of skin/transdermal drug delivery systems. An animal skin membrane that is sufficiently similar or close to human skin is needed to substitute human skin in ex vivo penetration and permeation, and topical bioavailability studies. Human skin is difficult to obtain and uniformity is difficult to maintain, since most of human skin comes from cadavers whose sex, age and genetic history are uncontrolled. Where as, animal skin is easier to obtain and is more uniform. Humans and animals have wide differences in the number of appendageal openings per unit area thickness of skin, structure and porosity of skin, and these factors clearly affect the percutaneous absorption of drugs. Only the progress in in vitro methodology with an appropriate animal model can resolve these limitations. It would be a simple mathematical exercise to predict the skin permeability in humans from animal experiments. If there is constant ratio between skin permeability in human to animal, independent of the drug under study. If correlation can be established for drugs with different physicochemical properties, experiments using appropriate animal skin will be more reliable in the developmental stage of skin/transdermal drug delivery systems. It appears that hairless guinea pig and Brattleboro rat are to be good animal models for skin/ transdermal drug delivery systems, where as snake appears not to be a good model to evaluate permeation of drugs across skin (unpublished data). In reviewing the studies comparing transdermal absorption of drugs between animals and humans, care must be taken to ascertain the influences of methodology and model might have on the data, and utmost care must be taken to avoid any misinterpretation or wrong conclusions. According to the literature available no consensus is available regarding animal models which truly reflect human skin. |
| |
|
Transdermal drug delivery systems have following advantages over conventional drug delivery.
|
| |
|
Advantages 27-29
|
| |
| 1. They can avoid gastrointestinal drug absorption difficulties caused by gastrointestinal pH, enzymatic activity and drug interactions with food, drink and other orally administered drugs. |
| |
| 2. They can substitute for oral administration of medication when that route is unsuitable, as in case of vomiting and diarrhea. |
| |
| 3. They avoid the first-pass metabolism and avoid drug deactivation by liver enzymes. |
| |
| 4. They are non-invasive so avoiding the inconvenience of parenteral therapy. |
| |
| 5. They provide extended therapy with a single application, improving compliance over other dosage forms, requiring more frequent dose administration. |
| |
| 6. Drug therapy may be terminated rapidly by removal of Transdermal drug delivery systems from the surface of the skin. |
| |
| 7. They are easily and rapidly identified in emergencies (e.g. unresponsive, unconscious or comatose patient) because of their physical presence, features and identifying markings. |
| |
| 8. They can be used for drugs with narrow therapeutic window. |
| |
|
Transdermal drug delivery systems have following disadvantages.
|
| |
|
Disadvantages
|
| |
| 1. The limitations of transdermal drug delivery are mainly associated with barrier function of skin, so it is limited to potent drug molecules. |
| |
| 2. Skin irritation or contact dermatitis due to drug, excipients and enhancers is another limitation. |
| |
|
Future of Transdermal Therapy
|
| |
| Ten years ago, the nicotine patch had revolutionized smoking cessation; patients were being treated with nitroglycerin for angina, clonidine for hypertension, scopolamine for motion sickness and estradiol for estrogen deficiency, all through patches. At that time, biotech medicinal was still being developed. During the past decade, the number of drugs formulated in the patches has hardly increased, and there has been little change in the composition of the patch systems. Modifications have been mostly limited to refinements of the materials used. The reason is the only a limited number of drugs fit the molecular weight, and potency requirements for transdermal absorption. |
| |
Conclusion
|
| |
| Successful transdermal drug application requires numerous considerations. Bearing in mind that the basic functions of the skin are protection and containment, it would seem exceptionally difficult to target the skin for drug delivery. However, with our greater understanding of the structure and function of the skin, and how to alter these properties, more and more new drug products are being developed for transdermal delivery. The properties of the drug, the characteristics of the transdermal device, selection of in-vivo model and the status of patient’s skin are all important for safe and effective drug delivery. The transdermal drug delivery system could be one day one of the best novel drug delivery system. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |
 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
|
| |
 |
 |
 |
| Figure 9 |
Figure 10 |
Figure 11 |
|
| |