Introduction
|
| |
| Aripiprazole is chemically 7-[4-[4-(2,3-dichloro) phenyl piprazine –1- yl] butoxy]-3,4-dihydro-1Hquinolin- 2-one and the molecular formula is C23H27Cl2N3O2. It is the sixth and most recent second- generation antipsychotic agent, used in the treatment of acute, manic and mixed episodes associated with bipolar disorder [1, 2]. Aripiprazole appears to mediate its antipsychotic effect primarily by partial agonism at the D2 receptor that has been shown to modulate dopaminergic activity in areas where activity may be high or low. It shows partial agonism at the 5-HT1A receptor and antagonist profile at the 5- HT2A receptor [3]. Only a few authors have described the validation methods for aripiprazole among which UV and HPLC methods have been developed for the drug in rat plasma, human plasma and pharmaceutical formulations [4, 5, 6, 7]. As these methods are expensive and not reproducible, we have made an attempt to develop a more precise, simple and economical spectrophotometric method with greater precision, accuracy and sensitivity for the analysis of aripiprazole in bulk and dosage forms. |
| |
EXPERMENTAL
|
| |
|
Materials
|
| |
| Aripiprazole bulk drug (Orchid Health Care, Chennai, India) and ethyl alcohol (S.D. Fine Chemicals Ltd., Mumbai, INDIA) were used for the study. |
| |
|
METHOD
|
| |
|
Selection of wavelength
|
| |
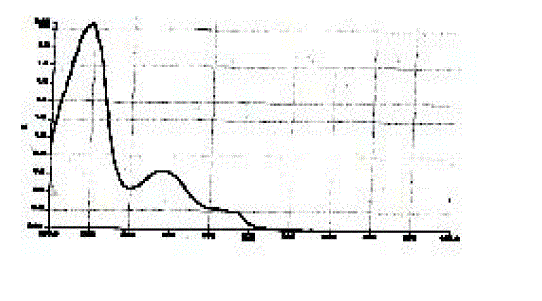
| The drug was found to have a maximum absorbance at 255.92 nm in ethyl alcohol and hence this λmax was selected for further studies (Figure 1). |
| |
|
Standard stock solution
|
| |
| A stock solution of 1000 µg/ml of aripiprazole was prepared freshly by dissolving 10.0000 mg of the drug in 10 ml of ethyl alcohol. |
| |
|
Sample solution
|
| |
| For the analysis of aripiprazole in tablets, a commercial brand, Arip-MT (Torrent Pharmaceuticals Ltd., Ahmedabad, India) of 10 mg strength was taken. Twenty tablets were weighed and powdered. The tablet powder equivalent to 10 mg of aripiprazole was weighed accurately and dissolved in 10 ml of ethyl alcohol. The solution was filtered through Whatman filter paper number 40 and the filtrate was diluted to 100 ml with water. An aliquot corresponding to 10 µg/ml was analyzed by the proposed method. |
| |
|
Procedure for calibration and assay
|
| |
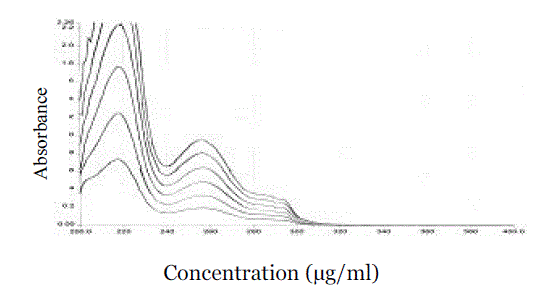
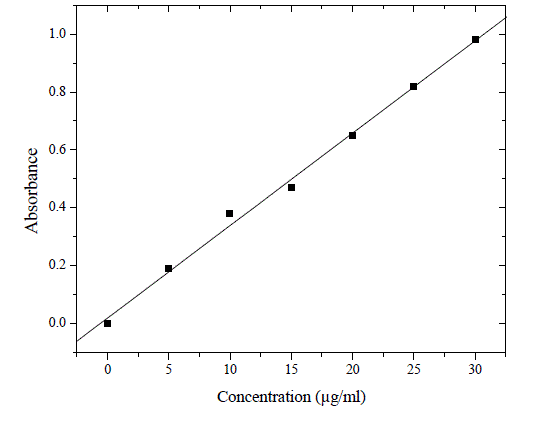
| The stock solution was diluted in such a way with double distilled water to get the required concentrations, 5, 10, 15, 20, 25 or 30 µg/ml. The absorbance was measured at 255.92 nm using a UV visible spectrophotometer (Perkin Elmer, Massachusetts 02451 USA) lambda 25 model with 1 cm matched quartz cell against ethyl alcohol as a blank (Figures 2 & 3). |
| |
|
Recovery study
|
| |
| Recovery experiments were carried out to study the accuracy, reproducibility and precision of the proposed method. Percentage recovery was calculated from the amount of the drug found (Table 1 & 2). |
| |
|
Statistical Analysis
|
| |
| Statistical analyses were carried out using Graph pad Prism 2.01 statistical software (Graph pad software, San Diego, CA, USA). Experiments were repeated for confirmation of results and to determine its reproducibility. The data of each experiment did not differ from each other significantly and hence all the data have been combined and means calculated. |
| |
RESULTS AND DISCUSSION
|
| |
| The proposed method for the determination of aripiprazole in solid dosage form was found to be precise, selective, rapid and economical. Aripiprazole exhibited maximum absorption at 255.92 nm and obeyed Beer’s law in the concentration range of 5-30 µg/ml. the proposed method for the determination of aripiprazole showed molar absorptivity of 0.74023 x 10-4, linear regression Y = 0.031X + 0.0156 with a correlation coefficient(r2) of 0.9995 (Figure 3). Similar results were obtained for granisetron hydrochloride, famiclovir, gatifloxacin in tablets and bulk dosage forms. A relative standard deviation of 0.330 % was observed on analysis of six replicate samples. Kumar et al., (2007) have described spectrophotometric, RP-HPLC and solvation (acetonitrile and 0.1 M HCl for dissolution and dilution respectively) methods for determination of aripiprazole. Our studies revealed a recovery percentage of 100.12 ± 0.52 %, which indicates that the developed method was simple, rapid and precise. Similar results were obtained in the studies of Kumar et al (2007). But most important of all, our method proves to be more economical than the published standard methods. |
| |
ACKNOWLEDGEMENT
|
| |
| The authors are grateful to Orchid Pharmaceuticals Private Limited, Chennai for providing authentic sample of aripiprazole, S.D. Fine Chemicals Ltd., Mumbai, India for providing solvents and reagents and Shree Samanvay Institute of Pharmaceutical Education and Research, India for providing necessary facilities and encouragement. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |









