Mahmood Masud Al Awfi*, Obaid Al-Hubaishi and Hani Al Qadhi
Department of Surgery, Oman Medical Speciality Board, Muscat, Oman
*Corresponding Author:
Mahmood Masud Al Awfi
Department of Surgery
Oman Medical Speciality Board
Muscat, Oman
Tel: 0096897150775
Email: mahmood.alawfi@gmail.com
Received date: January 15, 2018; Accepted date: January 16, 2018; Published date: January 18, 2018
Citation: Awfi MMA, Al-Hubaishi O, Qadhi HA (2018) Abdominal Wall Metastasis as the First Clinical Manifestation of Pancreatic Adenocarcinoma: A Case Report. J Univer Surg. Vol.6 No.1:3
Keywords
Pancreatic cancer; Metastasis; Abdominal wall metastasis; Pancreaticobiliary; Lymphadenopathy
Introduction
Pancreatic cancer has a poor prognosis with 6% overall 5-year survival rate. Among all cancers, it is considered as the fourth leading cause of death worldwide. Pancreatic cancer is usually asymptomatic in the early stages. The site of cancer in the pancreas determines the time of onset and nature of clinical presentation. These clinical manifestations are usually due to its spread to adjacent structures or distant metastasis. At the time of diagnosis, 90% of the patients have an overt metastasis [1,2]. Common metastases sites include; liver and peritoneal cavity. Lungs, bones and adrenal glands are less common. Other sites reported in the literature are testis, Stomach, pleura, thyroid, muscle, umbilicus, heart, kidney, appendix, prostate, skin and spermatic cord [3-7].
Three cases of abdominal wall metastases were reported in the literature and all were found post-operatively [8-10]. We present the first case of abdominal wall metastasis as the first clinical presentation in a virgin abdomen to our knowledge.
Case Presentation
A 55 years old gentleman with background of Diabetes Mellitus, Hepatitis C virus infection, cigarette smoking and alcohol consumption. He was referred to our hospital for evaluation of a para-umbilical mass. Patient noticed it two months prior to his presentation as it was painful on lifting heavy weight, otherwise he was asymptomatic. He had no past surgical history. On examination he was comfortable, not pale or jaundiced. He had bilateral inguinal lymphadenopathy. The mass was measuring 6 × 4 cm in the right upper side of the umbilicus with negative cough impulse, hard in consistency with distinct edges, non-reducible and mildly tender. Hernial orifices were intact and there was no ascites.
Investigations including hemoglobin level, liver function test, urea and electrolyte were within normal limits. These was followed with Computed Tomography (CT) scan, which showed a large midline soft tissue mass, measuring 8 × 4 × 6.6 cm with infiltrative speculated margins. It was centered at the anterior abdominal wall at the level of the umbilicus. It showed heterogeneous enhancement with multifocal small necrotic areas (Figure 1). It also showed focal ill-defined hypodense area in the pancreatic tail (Figure 2).
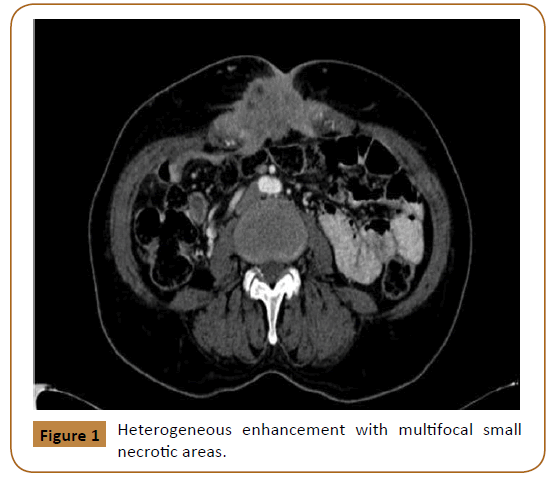
Figure 1: Heterogeneous enhancement with multifocal small necrotic areas.
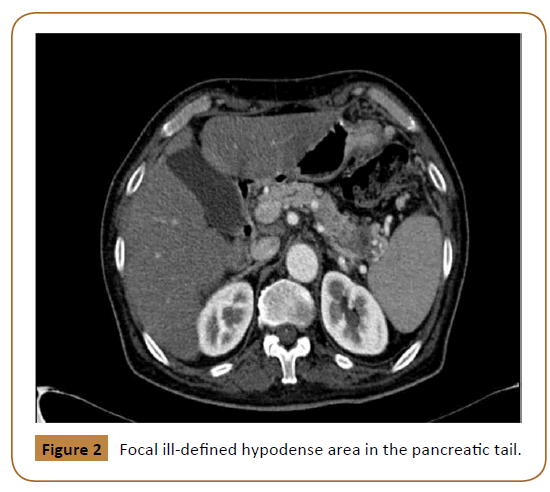
Figure 2: Focal ill-defined hypodense area in the pancreatic tail.
Subsequently, tumor markers were sent. Carcinoembryonic antigen (CEA) was 105.3 mcg/L (Normal Range in non-smokers is 0 to 2.5 mcg/L and in smokers is 0 to 5 mcg/L) and Cancer antigen 19-9 (CA 19-9) was 974 U/mL (A normal level is less than 37 U/ mL). Then, pancreatic MRI showed an ill-defined abnormal signal intensity lesion seen in tail of pancreas measuring approximately 2.5 × 2.1 cm in size. It contained two cystic areas measuring 9 × 7 mm and 7 × 5 mm. Main pancreatic duct was normal (Figure 3).
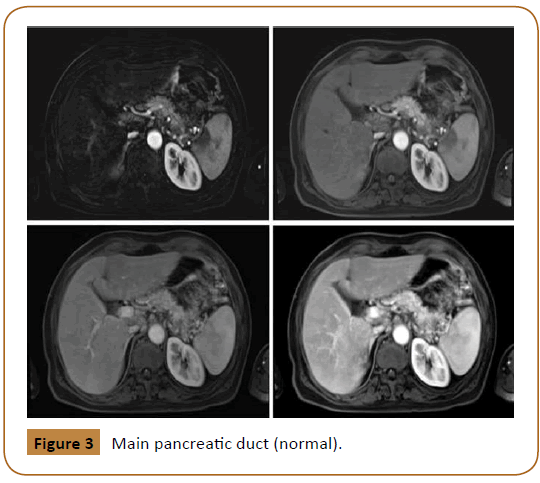
Figure 3: Main pancreatic duct (normal).
Staging CT-chest showed multiple pulmonary nodules most likely representing metastasis. Ultrasound guided biopsy of the abdominal wall mass was taken. The histopathology findings were consistent with metastatic poorly differentiated adenocarcinoma of pancreaticobiliary origin (Figure 4).
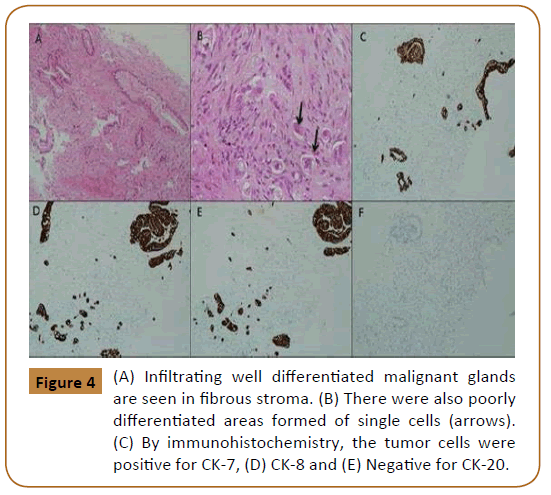
Figure 4: (A) Infiltrating well differentiated malignant glands are seen in fibrous stroma. (B) There were also poorly differentiated areas formed of single cells (arrows). (C) By immunohistochemistry, the tumor cells were positive for CK-7, (D) CK-8 and (E) Negative for CK-20.
This patient was discussed in a multidisciplinary team meeting and was planned for palliative care as there is no role for curative treatment in his condition.
Discussion
Pancreatic cancer can spread through lymphatic, hematogenous, transperitoneal route along with direct invasion. Metastatic route is highly determined by the site of malignancy within the pancreas. For instance, cancers of the body and tail spread more frequently through transperitoneal and hematogenous route than pancreatic head cancers [7,11].
Metastases to the abdominal wall can occur through different routes including; its rich arterial supply, veno-systemic circulation, or the lymphatic system. These are more prominent around the umbilical region. Moreover, umbilical invasion through embryonic remnant; i.e. umbilical ligament, have been described in the literature. In addition, abdominal wall metastases can occur through direct peritoneal extension and post-operative abdominal wall seeding [12-17].
In literature, abdominal wall metastases are mainly described in post-operative cases. Whereas umbilical metastases are described in a virgin abdomen as umbilical nodes or Sister Mary Joseph Nodule (SMJN) [8-10,14,18]. In our case the mass did not fall under any of these descriptions and we could not find any similar case reported in the literature.
An abdominal wall mass evaluation should aim to discriminate between primary and secondary lesions by clinical evaluation, laboratory and imagining studies. The clinical evaluation would give a clue about the nature of the lesion and its origin. In our patient the clinical examination suggested a malignant lesion. Therefore we proceeded with initial laboratory work up and a CT scan. The CT scan findings of the mass supported the clinical findings of suspected malignancy. It also led us to diagnose the pancreatic lesion.
CT scan findings were inconclusive thus we proceeded for a Pancreatic MRI, as it has been shown to be superior to CT scan in various studies [19-21]. This has added more diagnostic clues along with tumor markers that have shown an elevated CA19- 9 and CEA in this patient. This marker needs to be correlated with the clinical picture and the imaging findings to reach to an accurate diagnosis. CA19-9 is not highly specific for pancreatic cancer as it has been shown to be elevated in other benign pancreatic pathology and other malignancies such as bile duct, gastric and colorectal cancers [22]. Nevertheless it aids in the diagnosis and guides for further studies.
The histopathology examination with immunohistochemistry studies of the abdominal wall specimen has confirmed the diagnosis of metastatic poorly differentiated adenocarcinoma pancreaticobiliary in origin. The bio-markers of immunohistochemistry are useful in making an accurate diagnosis, estimating prognosis and making appropriate therapeutic decision.
Conclusion
In conclusion, abdominal wall mass could be the presenting symptom of metastatic pancreatic cancer and the literature do describe its potential to spread to wide verity of organs.
21743
References
- Hariharan D, Saied A, Kocher HM (2008) Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 10: 58-62.
- Muniraj T, Jamidar PA, Aslanian HR (2013) Pancreatic cancer: A comprehensive review and update. Dis Mon 59: 368-402.
- Borad MJ, Saadati H, Lakshmipathy A, Campbell E, Hopper P, et al. (2009) Skeletal metastases in pancreatic cancer: A retrospective study and review of the literature. Yale J Biol Med 82: 1-6.
- Matsumoto H, Yoshida Y (2015) Brain metastasis from pancreatic cancer: A case report and literature review. Asian J Neurosurg 10: 35-39.
- Delitala AP, Vidili G, Manca A, Dial U, Delitala G, et al. (2014) A case of thyroid metastasis from pancreatic cancer: Case report and literature review. BMC Endocr Disord 14: 6.
- Cormio L, Sanguedolce F, Massenio P, Di Fino G, Bruno M, et al. (2015) Testicular metastasis as the first clinical manifestation of pancreatic adenocarcinoma: A case report. J Medi Case Rep 9: 139.
- Yachida S, Iacobuzio-Donahue CA (2009) The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med 133: 413-22.
- Koga C, Tanemura M, Wada H, Kobayashi S, Marubashi S, et al. (2011) A case report of port-site metastasis of pancreatic cancer after laparoscope assisted distal pancreatectomy. Gan To Kagaku Ryoho 38: 2454-2456.
- Kameda R, Ueno M, Kobayashi S, Miyagawa K, Okawa S, et al. (2010) A case of abdominal wall metastasis from pancreatic cancer. Gan To Kagaku Ryoho 37: 1149-1152.
- Iuppa A, Petralia GA, Sciuto A, Romeo G (1996) Abdominal wall metastasis of pancreatic adenocarcinoma following laparoscopy. Ann Ital Chir 67: 265-9; discussion 269-270.
- Mao C, Domenico DR, Kim K, Hanson DJ, Howard JM (1995) Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma. Findings of 154 autopsies. Arch Surg 130: 125-34.
- Luz R, Leal R, Simões J, Gonçalves M, Matos I (2014) Isolated abdominal wall metastasis of endometrial carcinoma. Case Rep Obstet Gynecol : 505403.
- Gabriele R, Conte M, Egidi F, Borghese M (2005) Umbilical metastases: Current viewpoint. World J Surg Oncol 3: 13.
- Fábio C, Fernanda D, Carlos WS, de Araújo S (2004) Umbilical mass as the sole presenting symptom of pancreatic cancer: A case report. REV. HOSP. CLÍN. FAC. MED. S. PAULO 59: 198-202.
- Goshen E, Davidson T, Aderka D, Zwas ST (2006) PET/CT detects abdominal wall and port site metastases of colorectal carcinoma. Br J Radiol 79: 572-577.
- C. C. Nduka, J. R. T. Monson, N. Menzies-Gow, Mr A. Darzi (1994) Abdominal wall metastases following laparoscopy. Br J Surg 81: 648-652.
- Jacquet P, Averbach AM, Jacquet N (1995) Abdominal wall metastasis and peritoneal carcinomatosis after laparoscopic-assisted colectomy for colon cancer. Eur J Surg Oncol. 21: 568-570.
- Bai XL, Zhang Q, Masood W, Massod N, Tang Y, et al. (2012) Sister Mary Joseph’s nodule as a first sign of pancreatic cancer. World J Gastroenterol 18: 6686-6689.
- Semelka RC, Kelekis NL, Molina PL, Sharp TJ, Calvo B (1996) Pancreatic masses with inconclusive findings on spiral CT: Is there a role for MRI? J Magn Reson Imaging 6: 585-588.
- Sandrasegaran K, Lin C, Akisik FM, Tann M (2010) State-of-the-art pancreatic MRI. AJR Am J Roentgenol 195: 42-53.
- Miller FH, Rini NJ, Keppke AL (2006) MRI of adenocarcinoma of the pancreas. AJR Am J Roentgenol 187: W365-374.
- Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, et al. (2010) Tumor markers in pancreatic cancer: A European Group on Tumor Markers (EGTM) status report. Ann Oncol 21: 441-7.









