Key words
|
| |
| Gastro retentive system, Floating property, Swelling index, Hydrophobic polymer |
| |
INTRODUCTION
|
| |
| Oral controlled release (CR) dosage forms (DFs) have been developed over the past three decades due to their considerable therapeutic advantages such as ease of administration, patient compliance and flexibility in formulation. However, this approach is bedilled with several physiological difficulties such as inability to restrain and locate the controlled drug delivery system within the desired region of the gastrointestinal tract (GIT) due to variable gastric emptying and motility[1], to overcome this physiological problem, several drug delivery systems with prolonged gastric retention time have been investigated[1, 2]. Therefore, control of placement of a drug delivery system (DDS) in a specific region of the GI tract offers advantages for a variety of important drugs characterized by a narrow absorption window in the GIT or drugs with a stability problem[3]. These considerations have led to the development of a unique oral controlled release dosage form with gastro retentive properties. One of the most feasible approaches for achieving a prolonged and predictable drug delivery in the GI tract is to control the gastric residence time (GRT), i.e. gastro retentive dosage form (GRDF or GRDS). |
| |
| Floating systems or dynamically controlled systems are low-density systems that have sufficiently buoyancy to float over the gastric contents and remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. This results in an increased gastric retention time and a better control of the fluctuations in plasma drug concentrations. Many buoyant systems have been developed based on granules, powders, capsules, tablets, laminated films and hallow microspheres. This floating approach controls the placement of a drug and offers advantages for a variety of important drugs characterized by a narrow absorption window in the GIT or drugs with a stability problem. So, according to our best knowledge and literature surveys there were no reports or publications on floating tablet of aceclofenac using HPMCE5M and Eudragit RS 100. Hence in the present work is to design and develop the sustained release floating tablets of Aceclofenac using HPMCE5M and Eudragit RS 100 as polymers in order to enhance the absorption followed by improving bioavailability. |
| |
MATERIALS AND METHOD
|
| |
| Aceclofenac obtained as a gift sample from Amoli organics Pvt Ltd., Mumbai, Eudragit Rs100 was supplied from Bio - Gen extracts Pvt Ltd., Bangalore, HPMC E-5M gift sample obtained by Huzhou zhanwang pharmaceuticals co., Ltd, China, Poly vinyl pyrrolidone K30 Jiazuo yuanha fine chemicals co., Ltd, China, Sodium lauryl sulphate, Sodium bicarbonate were obtained from Qualigens Fine Chemicals, Mumbai, India, Talc and Microcrystalline cellulose were obtained from Loba chemie pvt.,ltd., Mumbai, India. All the chemicals and reagents required for the present experimental work are of analytical grade. |
| |
|
Method of preparation of Aceclofenac floating tablets [4]:
|
| |
| The aceclofenac floating tablets were prepared by blending the drug (aceclofenac), polymer (HPMCE5M / Eudragit RS 100) in different proportions respectively. To this sodium bicarbonate, sodium lauryl sulphate, poly vinyl pyrrolidine - K30 are added to mortar and pestle according to their geometric dilution and finaly make up the total weight (250mg) of tablet using micro crystalline cellulose. The powder was passed through sieve no.100. The obtained samples were collected and re triturated. To this required amount of talc is added and compressed finally. |
| |
| In the present work, 5 formulations (A1 to A5) floating tablets of Aceclofenac were prepared using variable concentrations of HPMCE5M and Eudragit RS 100 as shown in the Table no.01. |
| |
|
In – vitro characterization:
|
| |
|
a. Weight uniformity test [5]:
|
| |
| If the drug forms greater part of the tablet, any variation in the tablet weight obviously indicates a variation in the active ingredient this test resembles weight uniformity test. 20 tablets were selected at random and average weights were determined. Then individual tablets weighed and the individual weight was compared with the average. |
| |
 |
| |
| Average weight of tablets (X) = (X1+X2 +X3+…+ X20) / 20 |
| |
|
b. Hardness uniformity studies [5]:
|
| |
| The hardness of prepared formulation was measured by using Pfizer hardness tester. Five floating tablets were used for hardness uniformity studies. The hardness data used to calculate mean and standard deviation. |
| |
|
c. Thickness uniformity studies:
|
| |
| The thickness uniformity studies were carried out by using Vernier callipers. Five tablets were used for thickness uniformity studies and denoted in millimeter. The data obtained was used to calculate mean and standard deviation. |
| |
|
d. Friability (F):
|
| |
| The friability of the tablet was determined using Roche Friabilator. It is expressed in percentage (%). 20 tablets were initially weighed (W initial) and transferred into the friabilator. The friabilator was operated at 25 rpm per min for 4 mins (100 revolutions). The tablets were weighed again (W final). The % friability was then calculated by |
| |
 |
| |
|
e. Thickness and diameter [5]:
|
| |
| Tablet thickness is important for tablet packaging; very thick tablets affect packaging either in blisters or plastic containers. The tablet thickness is determined by the diameter of the die, the amount of fill permitted to enter the die and the force or pressure applied during compression. The thickness of the tablet may be measured manually or by automatic equipment. The thickness and diameter of the tablets was measured by Vernier Calipers. It is expressed in mm. |
| |
|
f. Content uniformity:
|
| |
| Twenty tablets were taken and amount of drug present in each tablet was determined. The tablets were crushed in a mortar and the powder equivalent to 100mg of drug was transferred to 100ml standard flask. The powder was dissolved in a suitable solvent and make up the final volume with the suitable buffer solution. The sample was mixed thoroughly and filtered through a 0.45μ membrane filter. The filtered solution was diluted suitably and analyzed for drug content by UV spectrophotometer, using buffer solution as a blank. |
| |
|
g. In vitro buoyancy / floating study:
|
| |
| In vitro buoyancy studies were performed for all the formulations. The randomly selected tablets from each formulation were kept in a 100ml beaker containing simulated gastric fluid, pH 1.2 as per USP. The time taken for the tablet to rise to the surface and float was taken as floating lag time (FLT). The duration of time the dosage form constantly remained on the surface of medium was determined as the total floating time (TFT). |
| |
|
h. Swelling Index [6]:
|
| |
| The swelling behavior of a dosage unit was measured by studying its weight gain. The swelling index of tablets was determined by placing the tablets in the basket of dissolution apparatus using dissolution medium pH 6.8 buffer at 37 ± 0.5°C. After 0.5, one, two, three, four, five, six, seven and eight hours, each dissolution basket containing tablet was withdrawn and blotted with tissue paper to remove the excess water and weighed on the analytical balance (Shimadzu, AX 120). The experiment was performed in triplicate for each time point. Swelling index was calculated by using the following formula. |
| |
|
i. Disintegration studies [7]:
|
| |
| Tablets were randomly selected and one tablet was introduced in each tube disintegration apparatus and placed in 1litre beaker containing water at 37°± 2°C and the time of disintegration was recorded. The study was done at room temperature without disc being added. |
| |
|
j. In vitro dissolution studies [8]:
|
| |
| The release rate of aceclofenac from floating tablets was determined using United States Pharmacopeia (USP) Dissolution Testing Apparatus 2 (paddle method). The dissolution test was performed using 900 ml of pH 1.2 HCL buffer for 2 hrs followed by pH 6.8 Phosphate buffer for 8hrs. A sample (5 ml) of the solution was withdrawn from the dissolution apparatus hourly and the samples were replaced with fresh dissolution medium. The samples were filtered through a 0.45μ membrane filter and diluted to a suitable concentration with of pH 1.2 HCL buffer for 2 hrs followed by pH 6.8 Phosphate buffer for 8hrs. Absorbance of these solutions was measured at 274 nm using a UV/Visible spectrophotometer. |
| |
|
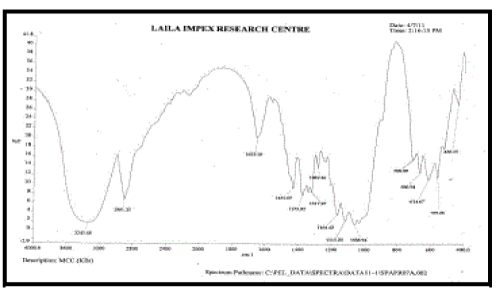
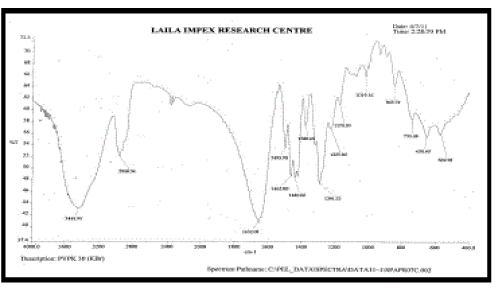
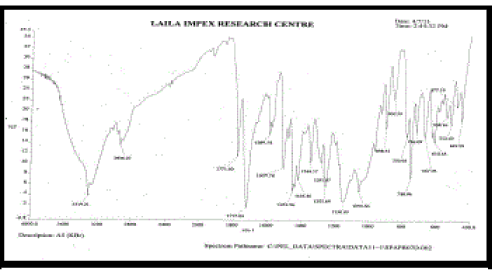
k. FT-IR spectra [9]:
|
| |
| Fourier transform Infra red analysis (FT-IR) measurements of pure drug, polymers and drugloaded floating tablets formulations were obtained using a model name BX- Perkinelmer System 200 FT-IR Spectrophotometer. The pellets were prepared on KBr press under hydraulic pressure of 150 kg / cm2, the spectra were scanned over the wave number range of 4000-400 cm-1 at an ambient temperature. |
| |
|
l. Drug release kinetics [10]:
|
| |
| The success of HPMCE5M with Eudragit RS100 in controlling the release of the drug was studied under the following heads to understand the order and probable underlying mechanism involved in the release pattern. To analyze the mechanism of drug release from the prepared formulations, the data obtained from in vitro release studies were subjected to Higuchi’s model, Zero order model and Korsmeyer’s model. |
| |
RESULTS AND DISCUSSION EVALUATION OF TABLETS:
|
| |
|
Weight Variation, Thickness, Hardness and Friability:
|
| |
| The results showed that weight variation, thickness were lying within limits. Because of variation in the compressional forces there is a slight variation in hardness of tablets. As the proportion of polymers increases the hardness of the tablets was found to increase in case of HPMC. Eudragit RS 100 tablets are less harder and thickest tablets. The friability loss was found to be within the limits in all the formulations. As the amount of polymer increased, the friability of the floating tablet was also found to increase. The results of Physical properties of aceclofenac floating tablets are given in Table No. 02 and the results revealed that the tablets are mechanically strong. |
| |
|
Buoyancy and total Flotation test:
|
| |
| From the results, it was observed that as the buoyancy lag time and the total floating time was studied for all the formulations. A1, A2, A3, A4 and A5 total floating time were found to be 8, 6.5 , 11, 12 and 10 hrs respectively as shown in Table no. 03. The formulations with hydrophilic polymer (A1, A2) showed less buoyancy lag time when compared to formulations with hydrophobic polymer (A3, A4). The formulation with combination of polymers (A5) showed optimum buoyancy lag time. for all the A3 and A4 formulations showed more total floating time when compared to A1 and A2 due to the presence of hydrophobic polymer which decreased the solubility. When compared in between A1 and A2 , A2 showed less total floating time. Thus with an increase in the concentration of the hydrophilic polymer total floating time was found to be decreased due to increase in the solubility. In case of A3, A4, A3 showed less total floating time. Thus with an increase in the concentration of the hydrophobic polymer total floating time was found to be increased due to decrease in the solubility. |
| |
| Results revealed that as the concentration of the hydrophilic polymer increases, the buoyancy lagging time decreases. The increase in the concentration of the hydrophobic polymer resulted in the increase of the buoyancy lag time. Thus polymer HPMC 55M and the combination of eudragit RS100 were found to have optimum floating characters for a longer period. |
| |
|
Disintegration time:
|
| |
| The disintegration time for all the formulations A1, A2, A3, A4 and A5 were found to be 140, 124, 185, 205 and 200 sec respectively as tabulated in Table no. 04. Formulations with hydrophilic polymer (A1, A2) disintegrated fastly when compared to formulations with hydrophobic polymer (A3, A4). This may be due to the greater water content present in the hydrophyllic polymer (HPMC). The higher volume of water sucked may have caused the disintegration of A1, A2 formulation quickly. |
| |
|
Swelling thickness:
|
| |
| As tabulated in Table no. 05 the extent of swelling was found out by measuring the thickness of the tablet before and after one hour stay of the tablet in pH 6.8 buffer at 37 ± 0.5°C. Formulation A2 tablets were found to swell more and formulation A4 tablets were swelling to lesser extent. |
| |
|
Drug content:
|
| |
| Drug content of all the formulations are within the acceptable range which shows the proper mixing of the drug with excipients as shown in Table no. 06. |
| |
|
In-vitro drug release:
|
| |
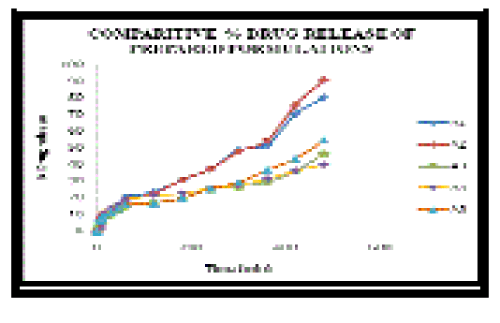
| In-vitro drug release study for all the formulations was conducted and tabulated in Table no. 07. Formulation with both the polymers (A5) showed sustained release. Formulations with hydrophilic polymer (A1, A2) showed high release of drug when compared to formulations with hydrophobic polymer (A3, A4) as shown in the Figure no.01. The hydrophilic polymer solubilized more and drug release was high. The hydrophobic polymer solubilized less which retards the drug release to a greater extent. Thus the HPMC with the combination of Eudragit RS 100 provide the optimum drug release. |
| |
|
Drug release kinetics:
|
| |
| From the data of drug release, it was found that, all the tablet formulations follow diffusion mechanism for the drug release. The Higuchi square root equation describes the release from systems where the solid drug is dispersed in an insoluble matrix and the rate of drug release is related to the rate of drug diffusion. Results revealed that all the prepared formulations follow Higuchi kinetics as shown in Table no.08. |
| |
|
FT-IR data:
|
| |
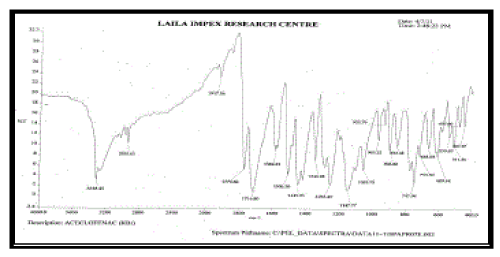
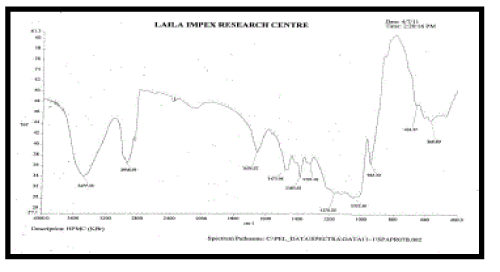
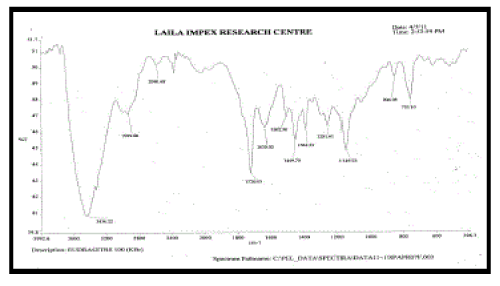
| FT – IR of pure aceclofenac, various polymers, other excipients and A5 formulation were recorded as shown in Figure no.02- 07. The aceclofenac present in the formulation A5 was confirmed by FT- IR. No predominant drug interaction was detected between drug and polymers along with excipients. Although there were some mild interactions in the wave number 1900-2100cm- 1. The region 3600 – 3200cm-1 was a stretching region of the functional group N-H, C-H of aromatic ring (3100 – 3000 cm-1), O-H (3200cm-1) and C- H alkenes (3020- 3100cm-1) and C- H of alkane. The Region 1500- 800cm-1, the weak interaction was noticed at 898.31, 852. All the peaks have appeared in pure aceclofenac and formulation A5 indicating no chemical interaction between aceclofenac and excipients. |
| |
CONCLUSION
|
| |
| The research was undertaken with the aim to formulate and evaluate the sustained release floating tablets of aceclofenac using HPMCE5M and Eudragit RS 100 as polymers. From results obtained, it was concluded that the formulation of sustained release tablet of aceclofenac containing a combination of both polymers (Eudragit RS 100, HPMCE5M) was taken as ideal or optimized formulation for 24 hours release as it fulfills all the requirement of sustained release dosage form. |
| |
|
ACKNOWLEDGEMENT
|
| |
| The authors are thankful to Mr. Ambati Brahma Reddy Research scientist, Glukem pharmaceuticals. Ms. Ramdevi Bhimavarapu and Karuna Priya Chitra Asst. Proffesors., for their valuable suggestions to fulfill this research work in a successful manner. |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
 |
 |
 |
 |
| Table 5 |
Table 6 |
Table 7 |
Table 8 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
|
| |















