Introduction
Sporadic, late-onset Alzheimer’s disease (AD) is a complex disease influenced by both genetic and environmental factors. Many of these factors have been identified during last decades. However, little is known about how these factors interact.
Growing evidence point the lysosomal aspartyl protease cathepsin D (catD) in AD-related processes as the activation of the endosomal/lysosomal system [1,2] and the cleavage of the amyloid precursor protein into amyloidogenic components [3]. Neuropathological changes in Alzheimer’s disease (AD) are also associated with increased expression of Apolipoprotein E (ApoE) and catD in astrocytes [4].
Additional evidence of the involvement of catD in AD comes from genetics: a non-synonymous polymorphism in the catD gene has been proposed to be a major risk factor for AD. Exonic polymorphisms of the catD gene possibly influences pro-catD secretion and intracellular maturation of the enzyme, was associated with the risk for the development of AD [5,6,7,8] although this result was not replicated in some populations [9,10]. Others suggest that there might be a synergistic interaction between the the CatD T allele and the APOEepsilon4 allele in increasing the risk for developing AD [11,12]. Strikingly gender differences were found recently [13] supporting the idea that this polymorphism confers an increased risk for AD in men but not in women.
Nevertheless, the enzymatic activity of serum catD had never been assessed in patients with dementia. We aimed to study the activity of serum catD in different stages of AD as well as in patients with Mild Cognitive Impairment (MCI) and Vascular Dementia (VD) in order to evaluate if this parameter could be considered a potential biomarker for AD.
Subjects and Methods
This project has been approved by the Research and Ethics Committees of the Hospital Universitario Central de Asturias (HUCA). Informed consent was obtained from all individuals or their guardians.
All individuals assessed at the HUCA-Dementia Unit from January 2003 to December 2005 meeting the inclusion criteria were invited to join the study.
Patients
Inclusion criteria
Patients suffering from one of the next conditions: MCI according to Petersen’s criteria [14], AD according to the NINDS-ADRA criteria [15], and VD according to the NINDS-AIREN criteria [16].
Exclusion criteria
Patients suffering from other conditions causative of cognitive impairment including cancer, hydrocephalus, infectious, metabolic or toxic disease. Patients with renal or hepatic dysfunction (often associated with abnormal levels of plasmatic proteins).
Controls
Individuals without any central nervous system disorder and ageing more than 60 years old were included as controls. Controls were recruited among healthy people matching the age of patients, mainly the spouses of the patients included, or patients studied in our department for peripheral nervous system diseases.
Clinical Assessment
All patients were studied with neuroimaging and full neuropsychological assessment following the AAN recommendations [17]. Neuropsychological assessment included the “Test Barcelona Abreviado” [18] in all patients as well as other tests depending on each patient’s profile. The Barthel Index [19] was also performed in all patients.
ApoE genotype was determined in all cases of AD: genomic DNA was obtained from blood following a salting-out method [20] and ApoE genotyping was performed as previously described [21].
The staging of the disease was performed following the GDSFAST criteria [22]: mild AD: GDS4, moderate AD: GDS5 and severe AD: GDS6.
Immunoassay
Plasma sample acquisition, storage and laboratory conditions were identical for all specimens used in the study. We used the MBL kit for cathepsin-D activity (Woburn, MA) which is a fluorescence-based assay that measures the free AFC (amino- 4-trifluoromethyl coumarin) released after cleavage of the preferred cathepsin-D substrate sequence RGFFP labelled with AFC. To this end serum samples were added to the synthetic substrate in fluorescence validate black 96-wells microtiter plates (BD FalconTM, Becton Dickinson) and incubated at 37ºC for 2 hours. Fluorescence was quantified in a Cary Eclipse Fluorescence Spectrophotometer (Varian Ibérica S.A., Madrid) using a 328 nm excitation filter and 460 nm emission filter. Results were expressed by the relative fluorescence units (RFU) per ml of serum.
Statistics
A Chi-square test was performed to study gender distribution differences between controls and patients. A T-Student test was performed to assess differences in age between controls and patients. ANOVA tests were performed to asses the differences in catD activity between groups. Chi-square tests were applied to assess whether catD activity is associated to the fact of being carrier of the E4 allele. Finally, the Mann–Whitney U test was used for assessing differences related both to gender and ApoE genotype.
Results
Sample description
We studied 40 controls and 149 patients. Sixteen patients suffered from MCI, 25 from VD and 108 from AD. Patients with AD were in different stages: 52 mild AD, 38 moderate AD and 18 severe AD. The mean age was 77,04 (std. dev.: 8,38) and there were no significant differences between groups (p=0.744). One hundred and 2 subjects were female and 47 male; there were no significant differences between groups (p=0.216), although in patients with AD there were a higher rate of female subjects than in other groups. Finally, 38% of patients with AD were carrier of the ApoE4 allele.
CatD activity
The activity of catD was similar in all groups (Table 1). The mean activity in controls was 659,83 units/ml. There were no significant differences between groups (p=0.252).The higher activity was found in patients with VD (706,99 units/ml) and the lower in patients with severe AD (587,35 units/ml) though this difference was not significant (p=0.105). There is no correlation between the severity of disease in AD and the catD activit
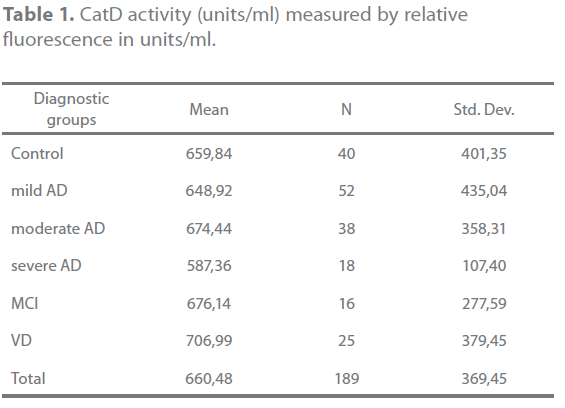
Table 1. CatD activity (units/ml) measured by relative fluorescence in units/ml.
Relation ApoE genotype-catD activity
When we studied the activity in patients with AD, those who were carriers of one E4 allele had lower activity (596,78 units/ ml) than those without E4 allele (702,95 units/ml), though this difference did not reach statistical significance (p=0.126). This trend was present for all disease stages, as shown in table 2.
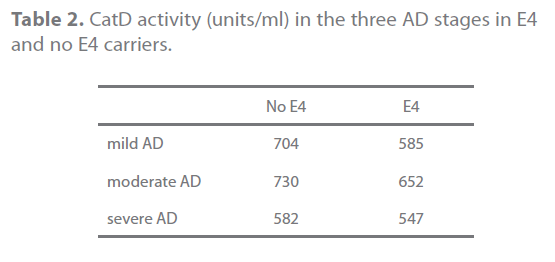
Table 2. CatD activity (units/ml) in the three AD stages in E4 and no E4 carriers.
On the other hand being carrier of two E4 allele did not associate with lower activity than being carrier of just one E4 allele (p=0,416).
Relation gender-catD activity
When we specifically compared the catD activity by gender, in both ApoE4-carriers and not carriers, we found a significant difference (p=0,037) only in the group of ApoE4 carriers: men showed a lower activity (mean: 542,27 units/ml – SD: ± 74,51) than women (mean: 639,36 units/ml – SD: ± 103,84) (Figure 1).
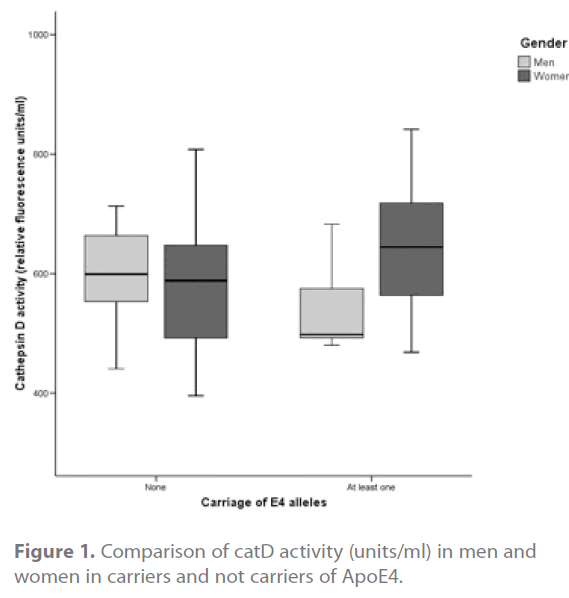
Figure 1. Comparison of catD activity (units/ml) in men and women in carriers and not carriers of ApoE4.
Discussion
Lysosomal impairment is involved in AD pathogenesis and can be detected not only in the CNS but also at a peripheral level [1,23]. This involvement may happen through catD. In fact catD seems to be involved in the proteolysis of ApoE and probably contributes to the generation of ApoE fragments previously implicated in AD pathology [24]. One of the studies presented at the 5th General Meeting of the International Proteolysis Society that address enzymatic mechanisms for producing neurotoxic beta-amyloid (Abeta) peptides describes the poor kinetics of BACE 1 for cleaving the wild-type (WT) beta-secretase site of APP found in most AD patients. They showed that cathepsin D displays BACE 1-like specificity and cathepsin D is 280-fold more abundant in human brain than BACE 1 [25].
We assessed the activity of serum catD in controls, AD, MCI and VD and did not found any significant association with the diagnosis of AD. However, there is a correlation with the genotype E4: at the three stages of the disease the activity is lower (not to the point of statistical significance) in those patients carriers of at least one apoE4 allele. This suggests that the ApoE genotype influences the activity of serum catD and in turn it can be more or less amyloidogenic. This finding is supported by genetic studies [11,12]. As the risk of AD in population is higher between E4 carriers one may infer that low catD activity might contribute to amyolidogenic deposits. In this line, Cathepsin D was found to be involved in the intracellular clearance of aggregatable Aβ [26].
Surprisingly, we also found a significant lower activity in men carriers of ApoE4, than in women or men who were not ApoE4 carriers. This finding also links with the results of genetic studies since the single nucleotide polymorphism rs17571 of the catD gene confers an increased risk for AD in men but not in women [13]. These facts support the hypothesis of gender-specific differences in the pathogenesis of AD [27].
Further studies, assessing the interaction between gender, ApoE and catD genes polymorphisms and catD activity are needed to fully understand their relation.
Conclusion
In the light of our results, the activity of serum catD does not seem to be a useful biomarker to distinguish between AD and VD or to monitor the progression of the disease, but these results confirm the possibility that the activity of serum catD in patients with AD is related to the ApoE genotype and gender and therefore might contribute to the pathogenesis of the disease only in a concrete subpopulation of patients: men carriers of ApoE4.
2310
References
- Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer’s disease.J Neurosci. 1996 Jan;16(1):186-99
- Kohnken RE, Ladror US, Wang GT, Holzman TF, Miller BE, Krafft GA. Cathepsin D from Alzheimer’s-diseased and normal brains. Exp Neurol. 1995 Jun;133(2):105-1
- Ladror US, Snyder SW, Wang GT, Holzman TF, Krafft GA.Cleavage at the amino and carboxyl termini of Alzheimer’s amyloid-beta by cathepsin D. J Biol Chem. 1994 Jul 15;269(28):18422-8
- Diedrich JF, Minnigan H, Carp RI, Whitaker JN, Race R, Frey W 2nd, Haase AT. Neuropathological changes in scrapie and Alzheimer’s disease are associated with increased expression of apolipoprotein E and cathepsin D in astrocytes. J Virol. 1991;65(9):4759-68
- Papassotiropoulos A, Bagli M, Kurz A, Kornhuber J, Förstl H, Maier W, Pauls J, Lautenschlager N, Heun R.A genetic variation of cathepsin D is a major risk factor for Alzheimer’s disease. Ann Neurol. 2000 Mar;47(3):399-403
- Mariani E, Seripa D, Ingegni T, Nocentini G, Mangialasche F, Ercolani S, Cherubini A, Metastasio A, Pilotto A, Senin U, Mecocci P. Interaction of CTSD and A2M polymorphisms in the risk for Alzheimer’s disease. J Neurol Sci. 2006 Sep 25;247(2):187-91
- Mateo I, Sánchez-Guerra M, Combarros O, Llorca J, Infante J, González-García J, del Molino JP, Berciano J. Lack of association between cathepsin D genetic polymorphism and Alzheimer disease in a Spanish sample. Am J Med Genet. 2002 Jan 8;114(1):31-3
- Schuur M, Ikram MA, van Swieten JC, Isaacs A, Vergeer-Drop JM, Hofman A, Oostra BA, Breteler MM, van Duijn CM. Cathepsin D gene and the risk of Alzheimer’s disease: A population-based study and metaanalysis. Neurobiol Aging. 2009 Nov 18. [Epub ahead of print]
- Sun Y, Shi JJ, Zhang SZ, Tang MN, Han HY, Guo YB, Ma C, Liu XH, Li T. The C224T polymorphism in the cathepsin D gene is not associated with sporadic Alzheimer’s disease in Chinese. Yi Chuan. 2005 Mar;27(2):190-4
- Ingegni T, Nocentini G, Mariani E, Spazzafumo L, Polidori MC, Cherubini A, Catani M, Cadini D, Senin U, Mecocci P. Cathepsin D polymorphism in Italian elderly subjects with sporadic late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16(3):151-511.
- Li XQ, Chen D, Zhang ZX, Qu QM, Zhang JW. Association between cathepsin D polymorphism and Alzheimer’s disease in a Chinese Han population. Dement Geriatr Cogn Disord. 2004;18(2):115-9
- Davidson Y, Gibbons L, Pritchard A, Hardicre J, Wren J, Tian J, Shi J, Stopford C, Julien C, Thompson J, Payton A, Thaker U, Hayes AJ, Iwatsubo T, Pickering-Brown SM, Pendleton N, Horan MA, Burns A, Purandare N, Lendon CL, Neary D, Snowden JS, Mann DM. Genetic associations between cathepsin D exon 2 C-->T polymorphism and Alzheimer’s disease, and pathological correlations with genotype. J Neurol Neurosurg Psychiatry. 2006 Apr;77(4):515-7 characterization and outcome. Arch Neurol. 1999;56(3):303-8
- Albayrak O, Tirniceriu A, Riemenschneider M, Kurz A, Scherag A, Egensperger R. The cathepsin D (224C/T) polymorphism confers an increased risk to develop Alzheimer’s disease in men. J Gerontol A Biol Sci Med Sci. 2010;65(3):219-24.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999 Mar;56(3):303-8.
- Tierney MC, Fisher RH, Lewis AJ, Zorzitto ML, Snow WG, Reid DW, Nieuwstraten P. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer’s disease: a clinicopathologic study of 57 cases. Neurology. 19;38(3):359-64.
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDSAIREN International Workshop. Neurology. 1993;43(2):250-60
- Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC. Practice parameter: diagnosis of dementia (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2001;56(9):1143-53
- Peña-Casanova J, Guardia J, Bertran-Serra I, Manero RM, JArne A. Versión abreviada del Test Barcelona (I): subtest y perfiles normales. Neurologia 1997;12:99-111
- Mahoney FI, Barthel DW. Functional evaluation: The Barthel index, Maryland State Med J 1965; 14: 61-5
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNAfrom human nucleated cells. Nucleic Acids Res 16 (1988), 1215
- J.E. Hixson and D.T. Vernier, Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI, J Lipid Res 31 (1990), 545–548
- S. Auer and B. Reisberg, The GDS/FAST staging system, IntPsychogeriatr 9 (1997), 167–171.
- Haque A, Banik NL, Ray SK. New insights into the roles of endolysosomal cathepsins in the pathogenesis of Alzheimer’s disease: cathepsin inhibitors as potential therapeutics. CNS Neurol Disord Drug Targets. 2008 Jun;7(3):270-7
- Zhou W, Scott SA, Shelton SB, Crutcher KA. Cathepsin D-mediated proteolysis of apolipoprotein E: possible role in Alzheimer’s disease. Neuroscience. 2006;143(3):689-701
- Hook V, Schechter I, Demuth HU, Hook G. Alternative pathways for production of beta-amyloid peptides of Alzheimer’s disease. Biol Chem. 2008 Aug;389(8):993-1006.
- Hamazaki H. Cathepsin D is involved in the clearance of Alzheimer’s β-amyloid protein (1996) FEBS Letters, 396 (2-3), pp. 139-142.
- Ghebremedhin E, Schultz C, Thal DR, Rüb U, Ohm TG, Braak E, Braak H. Gender and age modify the association between APOE and AD-related neuropathology. Neurology. 2001 Jun 26;56(12):1696-701.








