Keywords
Leucas aspera, Sub-acute toxicity, Traditional medicine, Ethanolic extract, Phytotherapy.
Introduction
Leucas R.Br. (Lamiaceae) is a large genus of herbs distributed throughout the tropical regions of the whole world with two main centers, one in eastern tropical Africa, the other in Asia, with its largest diversity in India. About forty three species occur in India. [1] Some of the species which have been proved for their medicinal values are L. aspera, L. biflora, L. cephalotes, L. ciliata, L. lanata, L. lavendulaefolia, L. linifolia, L. martini cenesis, L. stelligra, L. urticaefolia and L. zeylanica. Leucas aspera (thumbai) grows as an herbaceous, muchbranched, erect or diffuse annual, 30 to 60 cm high weed on wastelands. The plant is used as an insecticide and indicated in traditional medicine for coughs, colds, painful swellings and chronic skin eruptions. In traditional medicine, the leaves find their use in chronic rheumatism and juice for skin eruptions. [2] Literature survey shows the plant to have anti-fungal, anti-microbial, anti-ulcer and hepatoprotective effects. The analgesic, antiinflammatory and anti-pyretic efficacies of Leucas aspera have also been proved. The root parts of Leucas aspera showed the plant to have antinociceptive, antioxidant and cytotoxic activities. Pharmacognostical evaluation of the plant collected from various regions of northern India has been reported. Some of the compounds isolated from Leucas aspera include a hydroxyl tetra triacontan-4- one, aliphatic ketones, nicotine, alpha-farnesene, alpha–thujene, menthol, amyl propionate and isoamyl propionate. [3] Baicalin and Baicalein isolated from the flowers of Leucas aspera were found to stabilize RBC membrane integrity in hypotonicity induced hemolysis. [4] Prostaglandin inhibitory and in vitro antioxidant activity guided fractionation of shoots of Leucas aspera yielded eight lignans and four flavonoids inclusive of acacetin, apigenin, chrysoeriol, coumaryl –D-glucoside, nectandrin B, meso- dihydroguaiaretic acid, macelignan. [5] Characterisation of leucasin isolated from L. aspera leaves for free radical-scavenging showed that leucasin has the ability to chelate metal ions and inhibit oxidation of lipids by breaking the chain reaction due to Fe3+. [6] Despite the use of the plant in traditional medicine for ages, there is limited scientific evidence regarding the safety and efficacy to support the continued therapeutic application of these herbal remedies. Our study was therefore undertaken to determine the toxicity profile of Leucas aspera on rats. To the best of our knowledge, there is no record in the literature of the toxicity profile of Leucas aspera. The acute and sub-acute toxicity data may be required to predict the safety and effects of long term exposure to a particular medicinal plant. This study therefore seeks to assess Leucas aspera for its toxic effects using hematology, serum chemistry, and histopathological changes as toxicity indices.
Materials and Methods
Plant material
The aerial parts of the plant Leucas aspera were collected from Kanchipuram district of Tamilnadu. It was taxonomically identified by Dr. N.Jayaraman, Director, National Institute of Herbal Science, Plant Anatomy Research Center, Tambaram, Chennai India. Voucher specimen (PARC/2007/362) has been deposited in our college herbarium for reference.
Preparation of plant extract
Shade dried and coarsely powdered aerial parts of Leucas aspera (1 kg) was subjected to successive, exhaustive cold maceration in solvents of increasing polarity (n-hexane, chloroform, ethyl acetate and ethanol) for 72, 48, and 24 h respectively. The solvents were filtered, distilled under vacuum and dried in a vacuum dessicator to obtain four different extracts which were utilized for further studies.
In vitro antioxidant studies
The ability of the various extracts to scavenge DPPH radicals in vitro based on the method of Sreejayan and Rao, 1996 [7] was studied and percentage inhibition calculated. Based on these results, the ethanolic extract of Leucas aspera (EELA) which exhibited maximum free radical scavenging activity was selected for in vivo efficacy studies.
In vivo efficacy studies
The anti-inflammatory and antioxidant efficacy of EELA in CFA (complete Freund’s adjuvant) induced arthritis was studied on female Wistar rats. [8]
In vivo toxicity studies
Animals
Female swiss albino mice (25-30g) were utilized for the acute toxicity studies performed in accordance with OECD 423 [9] guidelines. Male and female Wistar rats (125–150 g) were used for subacute toxicity studies (OECD 407). [10] They were reared at the animal house of Vel’s University. Animals were kept in rat cages and fed on commercial pellets (Hindustan Lever Ltd, Mumbai, India) and allowed free access to fresh water ad libitum. The experimental protocols were approved by the Committee for the purpose of Control and Supervision of experiments on animals (CPCSEA), New Delhi, India (290/CPCSEA/PH04/17.10.07).
Preliminary acute toxicity studies
Swiss albino mice were divided into two groups of three each. Vehicle control group received 0.2% CMC and second group 2000 mg/kg b.w. of EELA suspended in 0.2% CMC. Immediately after the dose, animals were observed for first 4 h and next 14 days to record mortality. Animals were also observed for the presence of toxic symptoms such as weakness, aggressiveness, refusal of food, loss of weight, diarrhoea, noisy breathing, and fluid discharge from eyes and ears. [11]
Sub-acute toxicity studies
The rats were divided at random into a control group and three experimental groups with six animals in each group. The vehicle control group received 0.2% Carboxy methyl cellulose (CMC), whereas the experimental groups received EELA (100, 200, and 400 mg/kg body weight, p.o.), administered by means of bulbed steel needle for 28 days. Body weights were recorded on days 1 and 28 of the experiment, and daily observations were made for physiological and behavioral responses.
Collection of blood and serum samples
Animals from sub-acute tests were fasted overnight after the dosage period, anaesthetized with ketimine, and then decapitated. Paired blood samples were collected into heparinized and nonheparinized tubes. The heparinized blood was used for hematological evaluation; the non-heparinized blood was allowed to coagulate, contents centrifuged, and the serum separated was analyzed for biochemical parameters.
Determination of hematological and serum biochemical parameters
The hematological and serum biochemical parameters were determined. Hematological parameters assayed included red blood cell (RBC) and white blood cell (WBC) counts inclusive of polymorpho nuclear leucocytes and lymphocytes, platelets, hematocrit, and hemoglobin (Hb) estimation. Erythrocyte indices (mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular hemoglobin (MCH)) were determined from values obtained from red blood cell (RBC) count, Hb concentration, and packed cell volume (PCV) values. Serum was assayed for glucose, cholesterol, creatinine, urea, asparate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and protein.
Histopathology
Immediately after collection of blood samples, animals were opened up and various body organs such as liver, lungs, heart, kidney, spleen and brain were removed, weighed individually, and fixed in 10% buffered formalin in labeled bottles. Tissues were processed routinely and embedded in paraffin wax. Sections of 5 μm thick were stained with hematoxylin and eosin (HE) and examined under the light microscope. Stomach was cut open and observed for the presence of gross morphological changes.
Statistical analysis
The results are reported as mean ± SD for body weights, hematology, blood biochemistry, and organ weight data. The significant differences were assessed using one-way analysis of variance (ANOVA) using SPSS software package. P values less than 0.05 were considered significant.
Results
Acute toxicity study
There was no mortality or toxicity observed after oral administration of EELA up to a dose level of 2000 mg/kg body weight.
Sub-acute toxicity studies
Effect of EELA on the general behavior of rats
The oral administration of EELA caused no noticeable change in the general behavior of the rats and there were no significant changes in body weight (Table I) or food intake of the rats as compared to the control group. Both the control and treated groups appeared relatively healthy during the period of study. There were no deaths reported in any of the groups.
| Weight |
Control |
100 mg/kg |
EELA
200 mg/kg |
400 mg/kg |
| Initial b.w.(kg) |
152±9.7 |
147±8.9* |
155±10.6 NS |
148±8.6* |
| Final b.w. (kg) |
302±11.5 |
295±12.1* |
288±14.2* |
289±10.5* |
| Heart (g) |
0.69 ±0.06 |
0.58±0.11** |
0.60±0.09** |
0.59±0.04** |
| Liver (g) |
4.52±0.52 |
4.19±0.41* |
4.92±0.60* |
4.82±0.45* |
| Lung (g) |
0.7±0.04 |
0.65±0.06** |
0.72±0.05* |
0.68±0.1* |
| Spleen (g) |
0.64±0.12 |
0 .69±0.04* |
0.71±0.06* |
0.66±0.05 NS |
| Kidney (g) |
1.09±0.3 |
1.01±0.04* |
1.96±0.2** |
1.42±0.05 NS |
| Testis (g) |
1.02±0.03 |
0.98±0.05** |
0.92±0.04** |
0.94±0.03** |
| Ovary (g) |
0.04±0.00 |
0.04±0.01 NS |
0.04±0.00 NS |
0.04±0.02 NS |
b.w- body weight. Data are expressed as mean ± SD., n = 6. *P <0.05, **P <0.0.1 and NS- not significant as compared to Group I.
Table I: Effects of EELA on body weight and relative organ weights of treated rats.
Effect of EELA on hematological parameters of rats
The hematological analysis (Table II) showed no significant differences in any of the parameters examined in either the control or treated groups of both sexes. All the values remained within normal limits throughout the experimental period.
Effect of EELA on biochemical parameters of rats
| Blood Indices |
Control |
100 mg/kg |
EELA200 mg/kg |
400 mg/kg |
| RBC (x106/mm3) |
4.98±0.41 |
4.95±0.43 NS |
4. 82±0.33 NS |
4.92±0.18 NS |
| Hemoglobin (g/dl) |
14.90±0.5 |
14.4±0.82 NS |
14.62±0.44 NS |
14.83±0.28 |
| PCV (%) |
44.67±2.1 |
43.17±2.23 NS |
42.17±2.56** |
42.17±3.19 NS |
| MCV (fl) |
84.78±4.7 |
87.64±7.03 NS |
87.63±3.26 NS |
85.89±7.63 NS |
| MCH (pg) |
28.69±1.7 |
29.20±1.99 NS |
30.41±1.26 NS |
30.19±0.92* |
| MCHC (g/dl) |
33.86±0.7 |
33.36±0.93 NS |
34.73±1.44 NS |
35.36±3.02 NS |
| Platelets (x105/µl) |
1.18±0.13 |
1.52±0.07** |
1.45±0.27* |
1.57±0.20** |
| WBC (x103/mm3) |
3.533±0.4 |
3.383±0.51 NS |
2.866±0.320** |
2.746±0.329* |
| PMNL |
50.67±1.5 |
49.00±6.13 NS |
47.33±2.34* |
45.50±4.04* |
| Lymphocytes |
47.00±0.6 |
48.17±4.58 NS |
48.83±1.47* |
50.67±3.01* |
Table II: Effect of EELA on hematological parameters of treated rats
EELA caused no significant changes in blood glucose, urea, plasma creatinine, total proteins, total bilirubin and cholesterol whilst there was a limited increase in the activities of liver marker enzymes ALT and AST in group IV animals (Table III).
| Parameters |
Control |
100 mg/kg |
EELA
200 mg/kg |
400 mg/kg |
| Glucose (mg/dl) |
102±13.4 |
90±10.6 * |
105± 18.7 NS |
113±15.52* |
| Cholesterol (mg/dl) |
72±2.1 |
78.11±9.6* |
80.23±8.12* |
82.64±5.2** |
| Protein (g/dl) |
5.10±0.40 |
5.23±0.43 NS |
4.82±0.32* |
4.98±0.35 NS |
| Bilirubin (mg/dl) |
0.63±0.19 |
0.73±0.24 * |
0.71±0.31 NS |
0.69±0.12 NS |
| Urea (mg/dl) |
30.67±4.3 |
37.67±4.3** |
38.00±5.6** |
34.50±4.68 NS |
| Creatinine (mg/dl) |
0.93±0.15 |
0.73±0.26 * |
1.05±0.38 * |
0.95±0.36NS |
| ALP (IU/L) |
61±4.42 |
63.17±7.6 NS |
57.17±5.4 NS |
70.0±4.68*** |
| AST (IU/L) |
84.00±5.2 |
90.33±9.55* |
89.50±6.09 * |
93.8±7.22*** |
| ALT (IU/L) |
69.83±7.4 |
73.33±6.4NS |
81.2±7.15 ** |
92.5±7.55 *** |
ALP- alkaline phosphatase, AST- aspartate aminotransferase, ALT-alanine aminotransferase. Mean ± SD, n = 6 . Comparisons were made between Group I and Groups II, III and IV. *P <0.05, ** P <0.01 and NS- not significant as compared to Group I.
Table III: Effect of EELA on biochemical parameters of treated rats
Histopathological Studies
Histopathological analysis has shown all tissues apart from liver of the treated groups to be morphologically similar to that of the negative control groups.
Liver-The normal liver showed polygonal hepatocytes with a rounded nucleus, arranged in cords with the portal tract exhibiting a normal structure. In the treated groups, rats of groups II and III also exhibit normal morphological features whilst that of Group IV showed mild focal portal inflammation with lymphocytes, the structure of hepatocytes being normal and intact with no necrosis (Fig 1a, 1b, 1c and 1d).
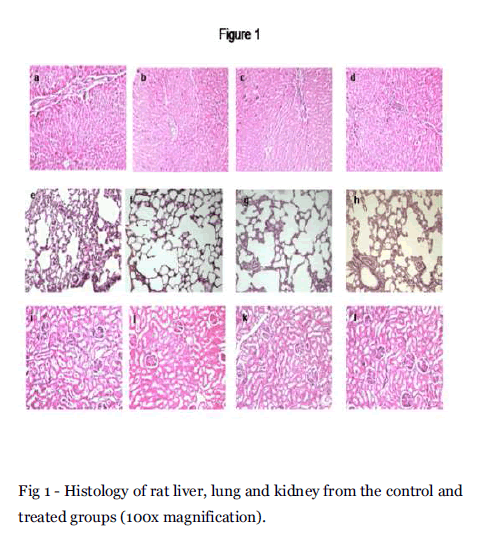
Fig 1 - Histology of rat liver, lung and kidney from the control and treated groups (100x magnification).
Lungs- Sections of lung tissue of control and treated groups showed normal alveoli (Fig 1e, 1f, 1g and 1h). Kidney-The kidney of the control group consists of normal cortex and medulla. The glomeruli and renal tubules namely the proximal and distal convoluted tubules exhibit a normal structure. The treated groups also exhibited a normal architecture indicating the absence of renal toxicity (Fig1i, 1j, 1k and 1l).
Heart –The histological features of control and Groups II, III and IV showed a normal myocardium at 100 x magnification (Fig 2a, 2b, 2c and 2d). Spleen – The spleen of the control rats showed the white pulp containing lymphocytes surrounded by a red pulp. The spleen of EELA treated groups also exhibited the same structural features (Fig 2e, 2f, 2g and 2h).
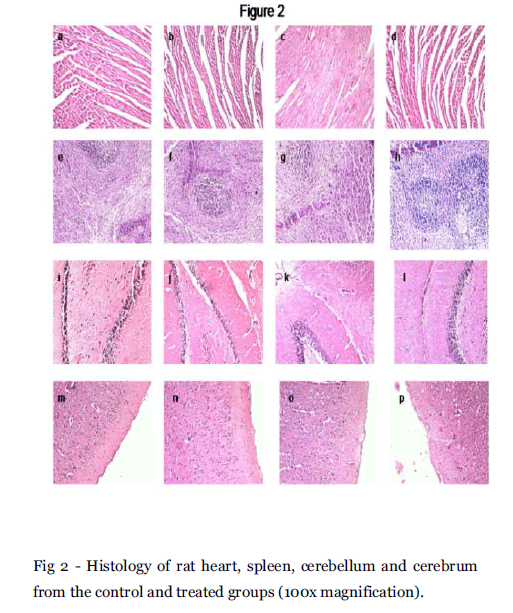
Fig 2 - Histology of rat heart, spleen, cerebellum and cerebrum from the control and treated groups (100x magnification).
Brain- Brain appears to be normal in the negative control group. Cerebrum and cerebellum are normal. The cerebellum (Fig 2i, 2j, 2k and 2l) and and cerebrum (Fig 2m, 2n, 2o and 2p) also had similar structural features.
Discussion
Herbal drugs have received greater attention as an alternative to clinical therapy and the demand for these herbal remedies has greatly increased recently. Their utilization is often based on long-term clinical experience. Despite the usage of plants in folk medicine over ages, only lately has pharmacology and toxicity of these plants begun to receive attention from scientists. With the upsurge in the use of herbal remedies in the last two decades, there is need for a thorough scientific evaluation of these medicinal plants. Hence to validate their claimed pharmacological properties and investigate their possible toxicity, preclinical toxicity studies were carried out on the ethanolic extract of Leucas aspera in mice and rat models.
In the present study, during acute toxicity evaluation, none of the animals died at the dose administered indicating that the LD 50 of EELA was much higher than 2000 mg/kg b.w. for the acute toxicity studies. Natural products usually do not present toxicity, and the arousal of toxic effects if any can be attributed to their use only when the effects occur immediately after administration. Further, there were no significant changes in food and water consumption. The determination of these parameters is important in the study of safety of a therapeutic product, as proper intake of nutrients and water are essential to the physiological status of the animals and to the accomplishment of proper response to the drug tested. [12] Body weight and organ weight changes serve as an indicator of adverse side effects since animals that survive cannot lose more than 10% of the initial body weight. [13] In the present study, in body weight evaluation, no significant difference was found, suggesting that EELA had no effect on the normal growth of rats, justifying the doses chosen.
This study has also shown that sub-acute treatment with the extract did not cause any change in hematological parameters. Hematological changes such as anemia are often accompaniments of bone marrow toxicity and analysis of blood parameters with respect to animal studies have a high relevance and predictive value for humans. [14] Further, the lack-of-effect on PMNL levels indicate that the extract may not have induced any inflammatory process since these cells are usually elevated in the course of inflammations. The anti-inflammatory role of EELA in adjuvant induced arthritis has already been established. [8]
In biochemical evaluation, there were no significant treatment related changes in blood glucose and serum cholesterol levels. Among the other parameters evaluated, AST and ALT are well known enzymes which serve as biomarkers capable of predicting toxicity. AST is present in a wide variety of tissues which includes heart, kidney, skeletal muscle and liver whereas ALT is primarily localized in the liver. [15] Both ALT and AST levels of groups II and III were not statistically different from that of control whereas the activities were slightly increased (P<0.001) in group IV animals. AST and ALT are often diagnostic of underlying cellular injuries. The analysis of these parameters is important since there are several reports of liver toxicity related to the use of phytotherapeutic products. [16] Histopathological examination of the selected organs from treated animals showed normal architecture in the groups II and III. Group IV animals exhibited mild infiltration of lymphocytes, but the hepatocyte structure was nomal and intact with no necrosis thus establishing the hepatoprotective role of EELA.
The insignificant difference in urea and creatinine levels between the treated groups and the control group probably indicate that the extract did not interfere with the renal capacity to excrete the metabolite. Indeed, creatinine is known as a good levels is only observed if there is marked damage to indicator of renal function. Any rise in creatinine levels is only observed if there is marked damage to functional nephrons. [17] Histopathological slides of kidney structure showed normal structural features suggesting the preserved renal integrity of EELA treated rats. Heart, lung, brain and spleen of the treated rats also did not demonstrate significant changes in morphology indicating the protective effect of EELA on these tissues. Further, stomach parts of treated animals did not show the development of ulcerative spots. The antiulcerogenic activity of L. aspera has earlier been recognized in the study of Reddy et al, 1992. [18]
Conclusion
Since, there were no significant adverse effects on the hematological and biochemical parameters, it may be concluded that the ethanolic extract of Leucas aspera did not induce any noteworthy damage to the vital organs. In conclusion, the present investigation demonstrates that at doses consumed in the traditional medicine, the ethanolic extract of Leucas aspera may be considered as relatively safe, as it did not cause either mortality or produce severe toxicological effects on selected body organs, biochemical indices and hematological markers of rats during the acute and sub-acute periods of study.
Acknowledgement
The authors are grateful to DST for financial grant.
Conflict of Interest: NIL
Source of Support: NONE
5612
References
- Khanam M, Hassan MA. A critical study ofnthe genus Leucas R.Br. (Lamiaceae) fromnBangladesh. Bangladesh J. Plant Taxonn2005; 12: 1-10.
- Kirtikar KR, Basu BD. Indian MedicinalnPlants. Dehradun, Lalit Mohan Basu, 1991,npp 2019-20.
- hydroalcoholic extract of Leucas asperanWilld (Link) on inflammatory markers innrheumatoid arthritis. Int J Green Pharmn2010; 4: 281-287.
- Manivannana R, Sukumar D. The RBCnmembrane stablisation in an in vitronmethod by the drug isolated from Leucasnaspera. Int J Appl Sci Engin 2007; 5: 133-n138.
- Sadhu SK, Okuyama E, Fujimoto H,nIshibashi M. Separation of Leucas aspera, anMedicinal Plant of Bangladesh, Guided bynProstaglandin Inhibitory and AntioxidantnActivities. Chem Pharm Bull 2003; 51: 595-n598.
- Meghashri S, Vijay Kumar H, Gopal S.nAntioxidant properties of a novel flavonoidnfrom leaves of Leucas aspera. Food Chemn2010; 122: 105ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂ110.
- Sreejayan N, Rao M. Free radical scavengingnactivity of curcuminoids. Drug Res 1996; 46:n169-171.
- Kripa KG, Chamundeeswari D, Thanka J,nUma Mageswara Reddy C. Modulation ofninflammatory markers by the ethanolicnextract of Leucas aspera in adjuvantnarthritis. J Ethnopharmacol 2011; 134:n1024-1027.
- OECD 2001. Test Guideline 423. Acute OralnToxicity ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
â Acute Toxic Class Method. In:nOECD Guidelines for the Testing ofnChemicals. Organisation for EconomicnCooperation and Development, Paris.
- OECD 1995. Test Guideline 407. RepeatednDose 28-day Oral Toxicity Study in Rodents.nIn:OECD Guidelines for the Testing ofnChemicals.nOrganisation for Economic Cooperation andnDevelopment, Paris.
- Saidu Y, Bilbis LS, Lawal M, Isezuo SA,nHassan SW, Abbas AY. Acute and sub-acutentoxicity studies of crude extract of Albizzianchevalieri Harms (Leguminosae). Asian JnBiochemistry 2007; 2: 224-236.
- Feres CAO, Madalosso RC , Rocha OA, LeitenJPV, Guimaraes TMDP, Toledo VPP,nTagliati CA. Acute and chronic toxicologicalnstudies of Dimorphandra mollis innexperimental animals. J Ethnopharmacoln2006; 108: 450ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂ456.
- Raza M, Al-Shabanah OA, El-Hadiyah TM,nAl-Majed AA. Effects of prolongednvigabatrin treatment on hematological andnbiochemical parameters in plasma, liver andnkidney of Swiss albino mice. ScientianPharmaceutica 2002; 70l: 135ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂ145.
- Hamid Rhiouani, Jaouad El-Hilalya, ZafarnH. Israili , Badiaa Lyoussia. Acute and subchronicntoxicity of an aqueous extract of thenleaves of Herniaria glabra in rodents. JnEthnopharmacol 2008; 118: 378ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂ386.
- Mukinda JT, Syce JA. Acute and chronicntoxicity of the aqueous tract of Artemisianafra in rodents. J Ethnopharmacol 2007;n112: 138ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂ144.
- Ozolua RI, Anaka ON, Okpo SO, Sylvester EnIdogun. Acute and sub-acute toxicologicalnassessment of the aqueous seed extract ofnPersea americana mill (lauraceae) in rats.nAfr J Complement Altern Med 2009; 6: 573nÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
â 578.
- Mukinda JT, Peter FK Eagles. Acute andnsub-chronic oral toxicity profiles of thenaqueous extract of Polygala fruticosa innfemale mice and rats. J Ethnopharmacoln2010; 128: 236ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
â 240.
- Reddy KM, Viswanathan S,nThirugnanasambandam P, Lalitha K. Antiulcernactivity of Leucas aspera Spreng.nAncient Sci Life 1992; 12: 257-260.








