Keywords
Acute leukemia; Lymphoblastic; Myeloblastic; Cytology; Immunophenotyping
Introduction
Acute leukemias (AL), heterogeneous group of clonal hematologic disorders characterized by malignant proliferation in the bone marrow of an abnormal cell clone of hematopoietic tissue and blocked at a specific stage of differentiation with expansion of immature cells (blasts) that may be present in the peripheral blood. Two broad types of AL are defined: lymphoblastic (ALL) and myeloblastic (AML), depending on the origin of the hematopoietic precursor.
In Europe and the United States, ALs represent 80% leukemia and about 35% of childhood cancers [1].
The current place of biology is fundamental because it allows to establish the diagnosis, to collect the factors of the prognosis in order to adapt treatment to predictable severity of the disease. In addition, it allows tracking of AL after introduction of treatments.
The objective of this work is to describe the cytological characteristics of acute leukemia cases collected at the Hematology Laboratory of Med VI University Hospital of Marrakech over a period of 2 years (January 2016-December 2017).
Materials and Methods
This is a descriptive and analytical retrospective study of 203 cases of patients in whom acute leukemia was diagnosed between January 2016 and December 2017. In our series, we included all patients of all ages, both sexes, with acute leukemia confirmed by myelogram. Blood samples were collected by venipuncture on tubes with EDTA K3 (ethylene diamine tripotassium tetracetic acid). Puncture of the bone marrow has been performed in adults with sternum and posterior iliac spine in children. The blood count was determined on the Sysmex XE-5000 i-automated system, and the blood and marrow smears were stained with MGG by the conventional manual method. For each patient, 2 independent readings of blood and marrow smears were assured and validated by 2 cytologists. The diagnosis of AL has been made when there is more than 20% of blasts in the bone marrow. Complement with myeloperoxidase (MPO) staining was done for all specimens. The use of immunophenotyping was done by flow cytometry mainly for MPO negative marrow infiltration, and uses the BD FACSCanto II flow cytometer. The data was collected from AL patients' files at the Hematology Laboratory of Med VI University Hospital of Marrakech. Data entry for the study was done on an Excel spreadsheet.
The statistical analysis consisted of a univariate descriptive method with calculation of percentages and averages. The various stages of our study were carried out respecting the anonymity and confidentiality of clinical and Para clinical data of patients.
Results
Cytological aspect
Morphological examination of marrow smears and reaction to myeloperoxidase, supplemented in some cases by immunophenotyping, classified leukemias into: AML in 54.47% of cases, ALL in 43.9% of cases, and in 1.63% of cases it was impossible to make an accurate diagnosis (Figure 1).
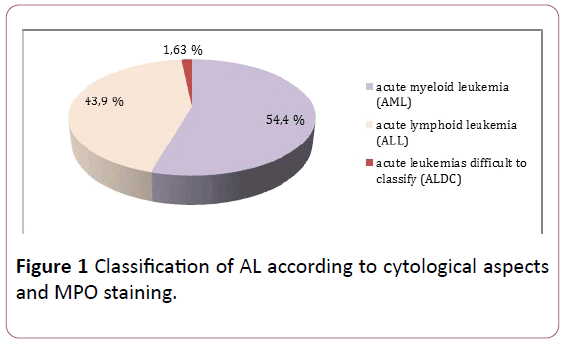
Figure 1: Classification of AL according to cytological aspects and MPO staining.
Age and sex
AML: The average age of the patients was 48 years old. Adults (18-80 years) were reached in 81.6% of cases and children (2-18 years) in 18.4% of cases, with an M/F sex ratio of 1.05.
ALL: The average age of the patients was 16 years old. Adults (18-80 years) were represented in 32.7% of cases and children (2-18 years) in 67.3% of cases (sex ratio M/F: 1.2) (Figure 2).
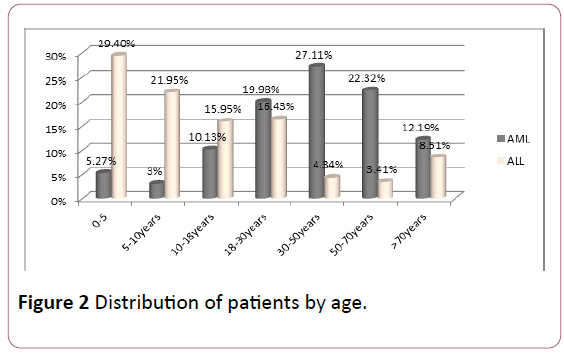
Figure 2: Distribution of patients by age.
The clinic
In our series patients; there is obvious variability of clinical manifestations, mainly represented by anemic syndrome (73% of cases), an infectious syndrome (52% of cases), whereas a syndrome of complete spinal cord failure was present in only 25% of cases (Figure 3).
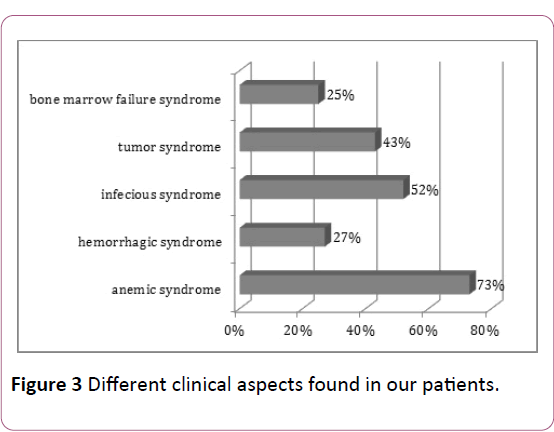
Figure 3: Different clinical aspects found in our patients.
Hemogram
In agreement with the clinical signs; the hemogram’s results show an attack of the 3 hematological lineages with a predominance of a normochromic normocytic are generative anemia (86% of cases), and major leukocytosis (>100000/mm3) in 76% of cases (Figure 4).
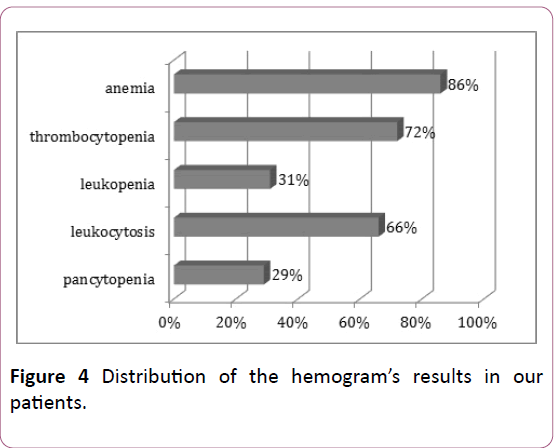
Figure 4: Distribution of the hemogram’s results in our patients.
The blood smear
Examination of the blood smear had established the white blood cell count and contributed to the classification of ALs according to the FAB group. The separation between AL subgroups was based on:
• The assessment of the percentage of blasts in the marrow.
• The type of blasts.
• The absolute count of blood monocytes.
• Circulating blasts were present in 86% of children and 72% of adults.
• The level of circulating blasts ranged from 4% to 90%.
Myelogram
AML: The study of the different types according to the FAB classification showed a predominance of AML4 and AML2: 38.8% and 25.37% respectively (Figure 5).
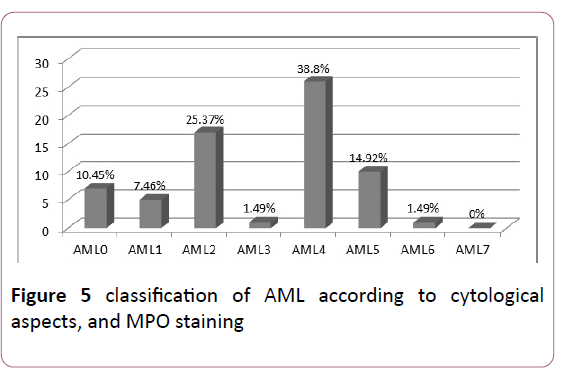
Figure 5: classification of AML according to cytological aspects, and MPO staining.
ALL: The complementary flow cytometry study of the cytological aspects evoking lymphoblastic leukemias, classify ALLs according to EGIL's classification, thus type B was predominant: 76% of ALLs (Figure 6).
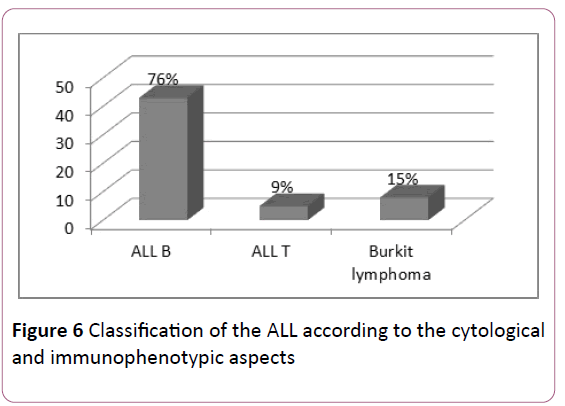
Figure 6:Classification of the ALL according to the cytological and immunophenotypic aspects.
Discussion
In a study published in 2009 by the regional register of malignant hemopathies of Lower Normandy in France over a period of 8 years (1997-2004), the average age for ALL was 25 years against 63 years for AML [2].
In 2011, a retrospective study conducted at the Hematology Laboratory of Ibn Rochd University Hospital in Casablanca, Morocco over a period of 4 years (2004-2007) reported an average age of 38 years for AML and 21 years for ALL [3].
In Brazil, the Rego and al study in the state of Piauí, between 1989 and 2000, reported an average of 9 years for ALL vs. 34 years for AML [4] (Table 1).
| Comparison |
UH Casablanca (Morocco) |
Lower Normandy (France) |
Brazil |
Our series |
| Average age ALL |
21 years |
25 years |
9 years |
16 years |
| Average age AML |
38 years |
63 years |
39 years |
48 years |
Table 1 Comparison by average age in our series and other series of the literature.
In our series of patients, there is a male predominance with a sex ratio M/F at any age at 1.12.
The distribution of cases by sex was comparable to that of the different series, and confirms the male predominance also found in the literature [5,6] (Table 2).
| Comparison |
Madagascar |
valence (France) |
Tunisia |
Brazil |
India |
Our series |
| Sex-ratio |
1.38 |
1.26 |
1.27 |
1.48 |
2.1 |
1.12 |
Table 2: Comparison of sex ratio in our series and other series of literature.
In our series, 73% of cases presented an anemic syndrome at diagnosis, the same result found by Sintha et al. (71%). In a study conducted in the Tunisian center between 1998 and 2008 and involving 281 patients, 15% of patients had an hemorrhagic syndrome [7], whereas it was present in 21% of cases in a study conducted by Sintha and al in India [8].
In our patients; the infectious syndrome was present in 52% of the cases. In the series of Rego and al in Brazil [4], 58% of patients had an infectious syndrome at diagnosis, while Sintha and al in India reports a higher frequency of around 80%.
The association of all three syndromes was observed in 25% of cases. In the literature, this association is more frequent in ALL than AML, especially in children, in fact, 23% of ALL cases present the triad of medullary insufficiency syndrome at diagnosis [9].
Tumor syndrome is more common in ALL (almost constant) than in AML (50% of cases) [10], and it is the consequence of the leukemic tumor mass [11]. In our series, it was found in 43% of cases.
The majority of our patients had normochromic normocytic are generative anemia.
Leukocytosis is a major prognostic factor. The prognosis is more favorable when the leukocytosis is less than 100 000/mm3 [11,12]. In our series, LAs frequently presented in hyper-leukocyte form (66% of cases) while leucopenia was found in only 31% of cases.
White blood cells were greater than 100,000/mm³ in 66%, this frequency is higher than those reported in the Sintha and al series in India (8.3%) [8], and in the Tunisian series (14%) [13].
Acute leukaemias can be complicated by disseminated intravascular coagulation (DIC), aggravating thrombocytopenia. Indeed, blast cells are rich in thromboplastinic substances that activate the extrinsic pathway of coagulation, which explains the frequency of DIC in leukemia with a greater procoagulant activity of the myeloblasts notably during promyelocytic acute leukemias [14].
We noted severe thrombocytopenia with a risk of cerebral hemorrhage in 33% of cases. This frequency is comparable to that reported by Jmili and Al in Tunisia (35%) [13], while in the Sintha and al series only 11.7% of patients had severe thrombocytopenia [8].
The most important element for diagnosing AL on the blood count is the presence of circulating blast cells. The absence of blast cells does not mean absence of AL, but rather absence of blood invasion by blasts [15]. In our series the level of circulating blasts ranged between 4% and 90%.
In our series, the morphological examination of blood and marrow smears and the reaction to myeloperoxidase allowed to classify ALs in:
• 54.4% of AML.
• 43.9% LAL.
• 1.63% of cases difficult to classify according to the criteria of the FAB group.
The results obtained are consistent with those published in the various series of the literature (Table 3).
| Comparison |
UH Casablanca (Morocco) |
Tunisia |
Madagascar |
UH Valence (France) |
Our series |
| AML |
64% |
51% |
56% |
82.7% |
54.4% |
| ALL |
30% |
40% |
41% |
15% |
43.9% |
Table 3 Comparison of AML and ALL frequencies.
Acute myeloid leukemias (AML) represent 1% of cancers and 80% of acute leukemias of adults whose incidence is constantly increasing. In children, it represents only 10 to 15% of AL and it is rare before the age of 15 [11]. In our series AML is the most common type accounting for 54.4% of acute leukemias. In Lower Normandy [2], we find higher results, 72% of AL are AML. At the Valencia Hospital Center their frequency is even higher, reaching 82% [16].
Cytologically, the subtypes M1 and M2 are the most frequent representing approximately 30% and 20% respectively. AML6, although relatively rare (3 to 5%), accounts for up to 20% of secondary AL [10] (Table 4).
| Comparison |
UH Casablanca (Morocco)% |
Tunisia % |
Madagascar % |
UH Valence (France) % |
Europe % |
Mexico % |
India % |
Our series % |
| AML0 |
8 |
-- |
2 |
3,9 |
3 |
4.2 |
-- |
10.45 |
| AML1 |
31 |
12 |
25 |
12 |
15-20 |
3.2 |
9.1 |
7.46 |
| AML2 |
28 |
17 |
36 |
20.9 |
25-30 |
20 |
59 |
25.37 |
| AML3 |
6 |
15 |
8 |
8,5 |
5-10 |
35.5 |
2.3 |
1.49 |
| AML4 |
6 |
16 |
13 |
14.7 |
20 |
1.4 |
18.2 |
38.8 |
| AML5 |
5 |
15 |
8 |
17.5 |
2-9 |
10.8 |
11.3 |
14.92 |
| AML6 |
5 |
7 |
6 |
6.2 |
3-5 |
0.8 |
-- |
1.49 |
| AML7 |
1 |
1 |
2 |
-- |
3-12 |
0.4 |
-- |
-- |
Table 4 Comparison of the frequencies of the different sub-types of AML.
The cytological characters of AML do not provide information on the prognosis. Studies have shown that the complete remission rate was higher in the M1, M2 and M3 categories than in the M4, M5 and M6 forms, but these findings have not been shared by other authors [17]. At present, cytogenetics and molecular biology, with their considerable contributions, have modified prognostic classifications [18]. We have made a comparison of the frequencies of the different types of AML in our series with other series of the literature.
In children, ALL represent 75 to 80% of leukaemias [19] and 25% of total cancers [20]. They occur in 75% of cases before the age of 6 years [12].
L1 subtype is the most common form in children [21]. The two varieties L1 and L2 of ALL are not really distinct by a particular category of blast cells, but rather by different proportions of cellular elements that they may have in common [22]. The prognostic value of the L2 forms compared to the L1 forms has never been demonstrated [23]. The FAB L1/L2 subclassification is no longer of interest since immunological and molecular subclassifications exist.
Fifteen percent of ALL cases in our series were L3. This form, distinguished by a very particular cytoplasmic criterion of Burkitt cells, should be considered apart from conventional ALLs. Type L3 is infrequent in the world, both in adults (9.7%) and children (2-4%) but has a higher prevalence in African countries [24] (Table 5).
| Comparison |
UH Casablanca (Morocco)% |
Tunisia % |
Madagascar % |
Europe % |
India % |
our series % |
| ALL T |
9 |
56.4 |
11 |
80 |
39.5 |
9 |
| ALL B |
75 |
32.1 |
79 |
17 |
60.5 |
76 |
| ALL Burkit |
6.5 |
5.1 |
11 |
3 |
|
15 |
Table 5 Comparison of the frequencies of the different subtypes of ALL.
We made a comparison of the different sub types of LAL with those of the literature.
Limits
In our series of patients; the diagnosis of acute myeloid leukaemias is essentially based on the cytolgic appearance and MPO staining, not completed by immunophenotyping, which is an indispensable tool to confirm the diagnosis.
The absence of new techniques, including cytogenetics and molecular biology, which currently fall into the criteria of diagnostic and prognostic classification of LA, particularly in the evaluation of residual disease [25-29].
Conclusion
The diagnosis of ALs is primarily based on cytological and immunophenotypic criteria of bone marrow blasts. The contribution of immunophenotyping, then of cytogenetics and finally of molecular biology have made it possible to describe more and more entities.
In Morocco, AL’s prevalence is underestimated by the absence of regional centers for treating hematological malignancies for both children and adults. Thus the cytogenetic and molecular techniques must supplement those existing for a better management of the Moroccan patients.
24211
References
- Löwenberg B, Downing JR, Burnett A (1999) Acute myeloid leukemia. N Engl J Med 341: 1051–1062.
- Troussard X, Duchenet V, Cornet E, Mouchel D, Malet M, et al. (2009) Hematological malignancies: Incidence in Basse-Normandie, France, for 1997-2004. Rev Epidemiol Sante Publique p: 57.
- Nafil H, Tazi I, Faez S, Benchemsi N (2012) Profil cytologique des leucémies aigues à Casablanca. J Africain du Cancer 4: 79–83.
- Rego MF, Pinheiro GS, Metze K, Lorand-Metze I (2003) Acute leukemias in Piaui: Comparison with features observed in other regions of Brazil. Brazilian J Med Biol Res 36: 331–337.
- Maynadié M, Troussard X (2015) Epidémiologie des leucémies aigues. Rev Francoph des Lab pp: 29–33.
- Linet MS, Dores GM, Kim CJ, Devesa SS, Morton LM (2013) Epidemiology and hereditary aspects of acute leukemia. Neoplastic Diseases of the Blood. Springer New York pp: 199–212.
- Jmili BN, Senana SH, Khelif A, Saad A (2010) Leucémies aigues myéloides en Tunisie: Caractéristiques épidémiologiques et cliniques et classification OMS. J Africain du Cancer 2: 25–32.
- Sintha M, Muthuraman M (2016) A retrospective study of clinical and laboratory parameter of acute leukaemias. MedPulse Int Med J 3: 542–546.
- Longo DL, Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373: 1541–1552.
- Provan D, Baglin T, Dokal I, De-Vos J (2015) Oxford handbook of clinical haematology. Oxford University Press.
- Cossio MLT, Giesen LF, Araya G, Pérez-Cotapos MLS, Vergara RL, et al. (2016) Hoffbrands essential haematology. Wiley Blackwell 33: 282.
- Hoffbrand V, Moss P, Pettit J (2011) Acute Leukemias. Essential Haematology p: 388.
- Jmili NB, Aziz ABA, Nagara M, Mahjoub T, Ghannem H, et al. (2005) Profil épidémiologique et cytologique des leucémies aigués: A propos de 193 cas colligés au centre Tunisien. Rev Française des Lab pp: 23–28.
- Aboab J, Drouet T, Muresan IP, Alamowitch S (2013) Hémorragie cérébrale fatale révélant une leucémie aigue promyélocytaire avec CIVD et leucostase. Prat Neurol 4: 262–264.
- Tuzuner NN, Bennett JM (2013) Classification of the acute leukemias: Cytochemical and morphologic considerations. Neoplastic Diseases of the Blood. Springer New York pp: 213–239.
- Raidelet l (2011) Epidemiologie des leucémies aigues de patients drômois et ardéchois diagnostiquées au centre hospitalier de valence de 2005 a 2010. Université Joseph Fourier.
- Thomas X (2008) Nouvelles approches thérapeutiques des leucémies aiguës de l’adulte. Rev Fr des Lab pp: 55–65.
- Preudhomme C, Llopis L, Boissel N (2012) Classification et facteurs pronostiques des leucémies aiguës. EMC- Hématologie 13: 1–18.
- Wartenberg D, Groves FD, Adelman AS (2008) Acute lymphoblastic leukemia: Epidemiology and etiology. Acute Leuk Hematologi pp: 77-93.
- Longmore M, Wilkinson IB, Davidson EH, Foulkes A, Mafi AR (2010) Oxford Handbook of Clinical Medicine. 4th edn. p: 912.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton AG, et al. (1976) Proposals for the classification of the acute leukaemias French-American-British (FAB) co-operative group. Br J Haematol 33: 451–458.
- Schrappe M (2003) Prognostic factors in childhood acute lymphoblastic leukemia. Indian journal of pediatrics 70: 817-824.
- Breccia M, Latagliata R, Cannella L, Carmosino I, De-Cuia R, et al. (2009) Analysis of prognostic factors in patients with refractory anemia with excess of blasts (RAEB) reclassified according to WHO proposal. Leuk Res 33: 391–394.
- Vardiman JW (2010) The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: An overview with emphasis on the myeloid neoplasms. Chem Biol Interact 184: 16–20.
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, et al. (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 114: 937-935.
- Gatta G, Zwan JMV, Casali PG, Siesling S, Dei-Tos AP, et al. (2011) The RARECARE working group: Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 47: 2493-2511.
- Torpy JM, CassioLynm MA, Richard M (2009) Acute Lymphoblastic Leukemia. JAMA 301: 452.
- Wagner-Ballon IO (2015) Place de la biologie moléculaire pour le diagnostic et le suivi des leucémies aiguës. Rev Fr Lab 471: 29-33.












