Keywords
“5CG-Desiccator”, in vitro cultivation, Trypanosoma brucei brucei,
Introduction
African trypaosomiasis, also known as sleeping sickness in human and Nagana in animal, is a parasitic disease caused by various salivarian species of trypanosome, a protozoa whose complex life cycle revolves around a mammalian host and an intermediary vector, the tsetse fly. The bloodstream forms of trypanosome could change the antigenic character of their glycoprotein surface coat, a process that allow them to evade immune response [1]. This phenomenon which is often referred to as antigenic variation, has rendered the development of trypanosomal vaccine almost impossible leaving chemotherapy as the only viable alternative.
There is an urgent need for the development of new trypanocidal drugs as current treatments agents are scarce, toxic and several species have exhibited resistance to them [2,3,4]. In addition, there are no drugs in routine use to prevent transmission during blood transfusion [5]. In vitro culture techniques are require for screening new compounds and plant-derived drugs for their antitrypanosomal effect and will permit close study of the immunology as well as the biochemistry of the parasite even though a lot have already been established in this regard. This will be particularly useful to developing countries that heavily rely on costly orthodox medications.
At present most of the in vitro screening of plant extracts conducted locally employed shorter time (from 5 minutes to 1 hour) for studies [6,7]. This duration is not adequate for drug-parasite interaction and so results from such studies may lead to wrong or misleading conclusions. One way out of this is to develop techniques that allow for the cultivation of trypanosome in vitro for longer period of time.
Internationally, several scientists have developed in vitro culture techniques for various stages of the parasite in the presence of feeder cells [8,9] and in a variety of axenic media [10]. These techniques, however, use CO2 incubator, which is expensive and out of reach of researchers in developing countries. Not only that, such advanced equipment requires steady source of electricity and hence not handy for field work. There is need for a simpler and cheaper in vitro culture system that will overcome the barriers set by cost, the need for advance equipment and much reliance on electricity among developing nations. Earlier preliminary study in this laboratory shows improvement in the survival rate of trypanosome parasites when slight amount of laboratoryprepared CO2 was introduced into a culture bottle containing the parasite suspension when compared to control bottles exposed totally to air. Since most standard in vitro cultivation techniques for trypanosomes utilizes CO2 incubator [11,12], it is felt that pursuing this observation further could provide a promising outcome. In the present work, we have tried to improve the culture methods for Trypanosoma brucei brucei in order to address some of the above concerns
It is in view of these that we attempt to adapt a “5CGdesiccator” as a simple and less expensive in vitro cultivation system for T. brucei brucei .
Methods
Animals and parasite collection
Rats were obtained from the animal laboratory in Kaduna State University, Nigeria. The T. brucei brucei was obtained from Nigerian Institute for Trypanosomiasis Research, Kaduna and were maintained in the laboratory by serial passage.
Sourcing of various sera
Fresh blood samples of horse, goat, sheep and cow were obtained from main abattoir in Tudun Wada, Kaduna-Nigeria while human blood was collected from a healthy volunteer.
Chemical /Reagents
Diethylaminoethyl cellulose (DE52) and Tris base were from Sigma Chem. Co. (USA). Amino acids, Sodium Chloride, Sodium Dihydrogen Phosphate (NaH2PO4.2H2O), Disodium Hydrogen Phosphate (Na2HPO4.12H2O), Glucose, Antibiotics (ampicilline, streptomycin), Ethylenediamine tetraacetic acid (EDTA), Chloroform, Glycerol, Giemsa powder, Calcium carbonate (Ca2Co3) were all of analytical grade.
Preparation of Sera
Fresh blood samples from horse, sheep, goat, cow and human were collected in sterile centrifuge tubes. The tubes were allowed to stand for 5 minutes after which they were centrifuge at 2000 rpm for 10 minutes. The red cells settle down the centrifuge tube and the serum supernatant collected using a pasture pipette into separate storage sample containers and stored at -20°C until required.
Maintainance of Trypanosoma brucei brucei in laboratory rats
The parasite T. brucei brucei was maintained by regular checking of the parasitamia in infected rats using the Herbert and Lumsden method of counting parasite in the five squares of a 16 squares haemocytometer and once the parasitaemia is found to be massive in any of the infected rats, 2 to 3 drops of the infected blood from the animal tail was collected in a glass container and mixed with little phosphate buffer saline glucose and healthy animals were inoculated with 0.2 ml of the mixed solution. The procedure is repeated when it becomes necessary.
Isolation of blood stream trypanosome from infected rats
Infected rats were sacrificed after chloroform anesthesia and blood collected in syringe containing 0.2 ml 1% (w/v) EDTA solution in 0.1M phosphate buffer saline. Pure parasites were isolated from an infected blood using ion exchange chromatographic method of Lanham and Godfrey [13]. The resin, DE52 cellulose in phosphate buffer saline glucose was transferred into a 20 ml syringe whose outlet was blocked with little cotton wool. The 20 ml syringe was fixed to a retort stand and a small clean beaker was placed at the bottom of the syringe. Before the application of the blood, the column (a 20 ml syringe whose plunger has been removed) is equilibrated with phosphate buffer saline glucose (pH 8.0). Thereafter, 2 ml of infected blood from the animal with rising parasitaemia (between 30 to 60 parasites per microscopic field) was then added to the column and eluted with the same PBSG. By this procedure, the red cells stick to the resin while the parasites come down in the eluent as a thick white suspension. The column is continually washed until eluent is transparent again.
Daily monitoring of Parasitaemia in infected rats
The parasitaemia in infected animals was monitored using the rapid matching method of Herbert and Lumsden [14]. Briefly, tail of infected animal is punctures with needle and a drop (about 5 μl) of blood collected on a clean slide, covered with a cover slip and viewed under microscope at X400 magnification.
The number of parasites counted in a field of the microscope is then compared with standard table provided by Herbert and Lumsden from where the corresponding absolute number of trypanosomes per ml of blood is extrapolated [14].
Counting of parasite in suspension and culture media
Parasite densities in the suspension as well as in the culture media were determined as described previously [15]. This involves counting the number of live trypanosomes (as judged by their motility) from 5 squares of the red blood cells counting region of a New Improved Neubauer counting chamber. The parasite density is then calculated using the formula below:
Density (cells/ml) = Number counted x 50 x1000 x Dilution Factor employed.
Laboratory preparation of gaseous carbon dioxide
About five grams of calcium carbonate was transferred into a glass bottle after which 2M hydrochloric acid was pumped from a wash bottle (A) into the reaction glass unit (B). The gaseous carbon dioxide released was passed through rubber tubing into a deflated polythene gas reservoir (C) until it was fully inflated as shown in Figure 1. The chemical equation for the reaction is:
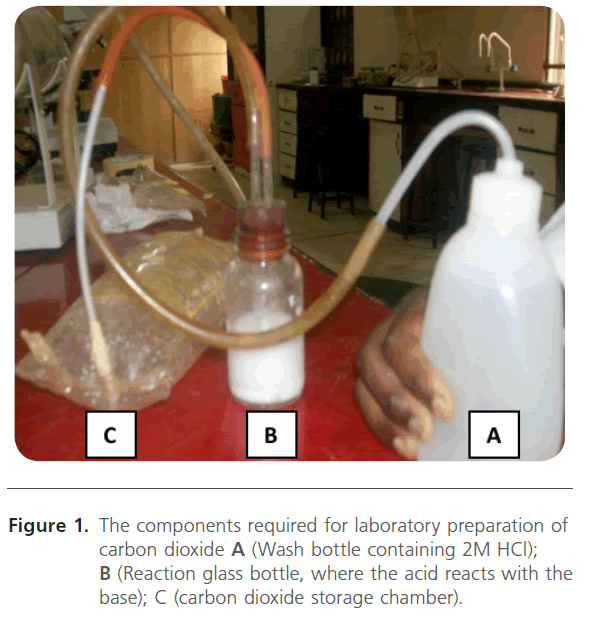
Figure 1. The components required for laboratory preparation of carbon dioxide A (Wash bottle containing 2M HCl); B (Reaction glass bottle, where the acid reacts with the base); C (carbon dioxide storage chamber).
CaCO3(S) + 2HCl(aq) → CaCl2(aq) + CO2(g) +H2O
Preliminary screening of various sera for in vitro survival of trypanosomes
Ten clean culture tubes (3 ml capacity) were labelled; cow, horse, goat, sheep and human in duplicate. Two ml of each heat inactivated serum was pipetted into the appropriate labelled cultured tubes. Five micro liter of antibiotic solution (to give an effective antibiotic concentration of 100μg/ml) and fifty micro liter of the pure parasite suspension (6 x 107 parasites/ ml) were added into all the cultured tubes with gentle shaking before they were incubated in a candle jar. The experiments were monitored at 6 hour intervals.
Study on the effect of various gaseous environments on the survival of trypanosomes
Exactly 50 μl of parasite suspension (8 x 104 parasite per bottle), supplemented with 0.5% glucose, and 10 μl of antibiotic mixture (streptomycin and penicillin, each at 10 μg/ml) were dispensed into 9 glass bottles (of 8 ml capacity) containing 200 μl of heat inactivated horse serum. Of the nine bottles, three (A) were plugged with cotton wool and kept under atmospheric air. The next set of three bottles were left open in a 2000 ml desiccators, little amount of carbon dioxide, prepared in the laboratory, was introduced to the desiccators and immediately covered and sealed with petroleum jelly to prevent gaseous exchange with the exterior of the desiccators. The last set of bottles was kept open in a candle-jar (a similar glass desiccator into which a burning candle is allowed to extinguished by itself). The candle-jar system, also referred to as high oxygen environment, has been estimated to contain 16-18% oxygen [16,17]. These setups were kept at room temperature (27-28 oC) and their densities determined for 18 hours at interval of 6 hours.
Study on the effect of various carbon dioxide concentrations in the 5CG-desiccator
Four desiccators of varying capacity were employed in this study. Three bottles containing culture mixture as described earlier were placed in each of the desiccators. Different amounts of laboratory prepared CO2 were added to three of the desiccators in three different concentrations (8, 20 and 50%) of the gas (within the desiccators). The fourth desiccator was left as control (0% CO2). Mean parasite densities were determined after 24 hours and compared.
Study on the effect of glucose on cultivation of Trypanosoma bucei brucei
Exactly 200 μl of horse serum were placed in eight wells of 96-well microtiter plate then 10 μl of antibiotic mixture and 50 μl of parasite suspension were added. The parasite density in each well is about 1.65 x 106 parasites/ml. The culture media in the wells adjusted into four sets of glucose concentrations; 10, 1.0, 0.1 and 0.01 % (w/v) and were incubated in 5% CO2 gased-desiccator (from where we coined the caption “5CG-Desiccator”) at 27oC. Each concentration was in duplicates. Parasite densities were determined from all the wells at 6 hours intervals for twelve hours.
Study on the effect of temperature on parasites in vitro survival
In this experiment four desiccators of identical capacity were used and all conditions (capacity of desiccators, 5% CO2, and initial parasite densities) were kept constant but the temperatures of cultivation were deliberately varied (20, 28, 30, and 37 oC). The ability of parasites to grow at these temperatures were observed by monitoring a decline in parasite densities after 24 hours.
Study on the effect of pH on in vitro survival of Trypanosoma brucei brucei
To each well of microtiter plate, 100 μl of heat-inactivated horse serum, 100 μl each of phosphate buffers (pH 6.0, 6.4, 6.8, 7.0, 7.2, 7.4) and Tris buffers (pH 7.8 and 8.0), 20 μl of parasite suspension and 10 μl of antibiotic solution (containing 100 μg/ml of penicillin and streptomycin) were added and mixed with the aid of pasteur pipettes. Each experiment was carried out in triplicate, incubated in 5CG-desiccator and parasite densities determined after 12 hr incubation period.
Study on the survival of Trypanosoma brucei brucei in various media/solutions
Eagle Minimum Essential medium (containing Amino Acids, Vitamins and Inorganic salts) as described by Eagle [18]; Amino Acid solutions (Eagle medium without vitamins) and Vitamin solution (Eagle media without Amino acids) are three laboratory prepared media that were screened for their ability to sustained T. b. brucei invitro. The experiments were conducted in triplicate against controlled solution (Phosphate Buffer Saline). For each experiment, 50 μl of parasite suspension (8 x 104 parasites/ml) was dispensed into 200 μl of the medium in wells of micro titre plates and the parasite density determined before and after incubation period of 24 hours.
Morphological observation of cultured parasite
Smears of the bloodstream parasite isolate and those of parasites cultivated under different CO2 concentrations were prepared on glass slide, allowed to dry in air and then fixed by dipping in methanol and stained with giemsa stain. Stained parasites were observed under the compound microscope using oil immersion.
Infectivity study of cultured Trypanosomes in mice
Infectivity studies were conducted to know if the cultured parasite still retain the ability to infect a host or not. Eight mice were divided into four groups of two mice per group. Each group was inoculated intraperitoneally with parasite cultured in different carbon dioxide and parasitaemia concentrations (0%, 8%, 20%, 50%) followed daily until the death of the animals.
Result and discussion
This study has successfully adapted a glass desiccator gassed with laboratory prepared 5% CO2 as an Invitro culture system for trypanosomes (5CG-Desiccator).
This study came as a result of our preliminary observation that when slight amount of laboratory-prepared CO2 was introduced into a culture bottle containing parasite suspension, parasite survived better than control bottles exposed totally to air. Since most standard in vitro cultivation techniques for trypanosomes used CO2 incubator [11,12], it is felt that pursuing this observation further could provide a promising outcome.
When the parasite T. brucei brucei was grown under different gaseous atmosphere, results (Figure 1) revealed that the parasite densities in reaction bottle of both the Candle-jar and the room-atmosphere dropped greatly in comparison with those exposed to laboratory-prepared CO2 after 12 hours of incubation. This difference in survival is attributable to the differences in the amount of carbon dioxide in each gaseous environment; the composition of the atmosphere itself is about 21 % oxygen, 78 % nitrogen, and less than 1 % carbon dioxide while the candle-jar system, is known to have high oxygen environment of between 16-18% oxygen [16,17]. The 5CG-desiccator system, however, employs 95 % air and about 5 % carbon dioxide. Further comparison was made of the capacity of the gas reservoir employed relative to the capacity of glass desiccator by setting up glass desiccators at different concentrations of carbon dioxide: the results of the effects of different concentrations (%) of the laboratoryprepared CO2 in culture bottles (Figure 2) shows that CO2 is very essential in the in vitro maintenance of the parasite T. brucei brucei. Even though the parasites grown for 24 hours under different CO2 concentrations proved infective (Table 1), higher concentrations of CO2, however, were found to suppress the growth and survival of the parasite (Figure 2), in addition to, affecting their morphologies when compared with bloodstream forms thus an excess of CO2 result in inhibiting growth and survival.
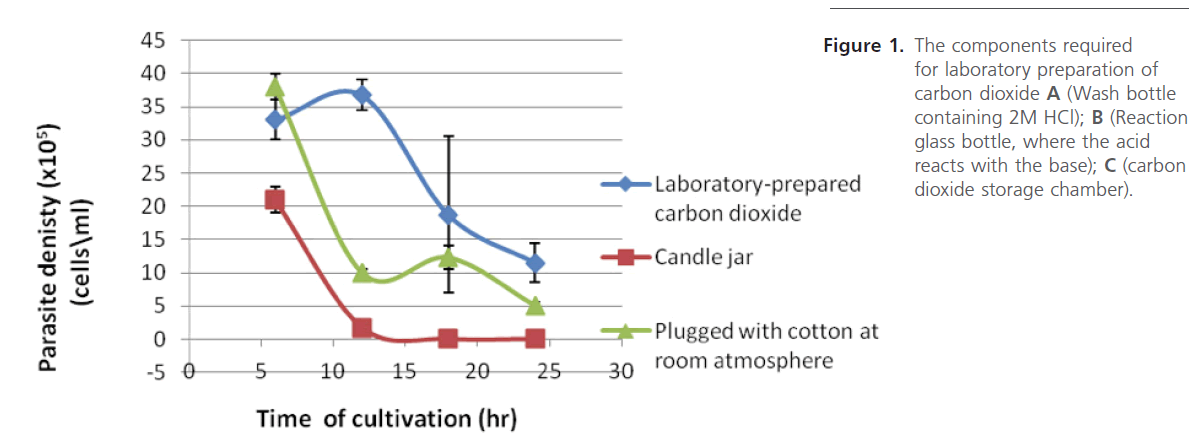
Figure 1: The components required for laboratory preparation of carbon dioxide A (Wash bottle containing 2M HCl); B (Reaction glass bottle, where the acid reacts with the base); C (carbon dioxide storage chamber).
Figure 2: Effect of different concentration of carbon dioxide on in vitro survival of T. brucei brucei.
Table 1. Infectivity potential of Trypanosoma brucei brucei cultured under different percent of CO2 in 5CGdesiccators.
Another major aspect of in vitro cultivation of trypanosomes is supplementing the culture media with glucose since trypanosomes rely on exogenous glucose as energy source [19,20]. Figure 3 shows that glucose concentration between 0.01 and 1.0 % (w/v) gave better results than that obtained with 10%. This range is thought to be only good for bloodstream forms and not the procyclic forms as the later are very sensitive to glucose at low levels (0.2-0.5%) and so must be grown in glucose-free medium [21].
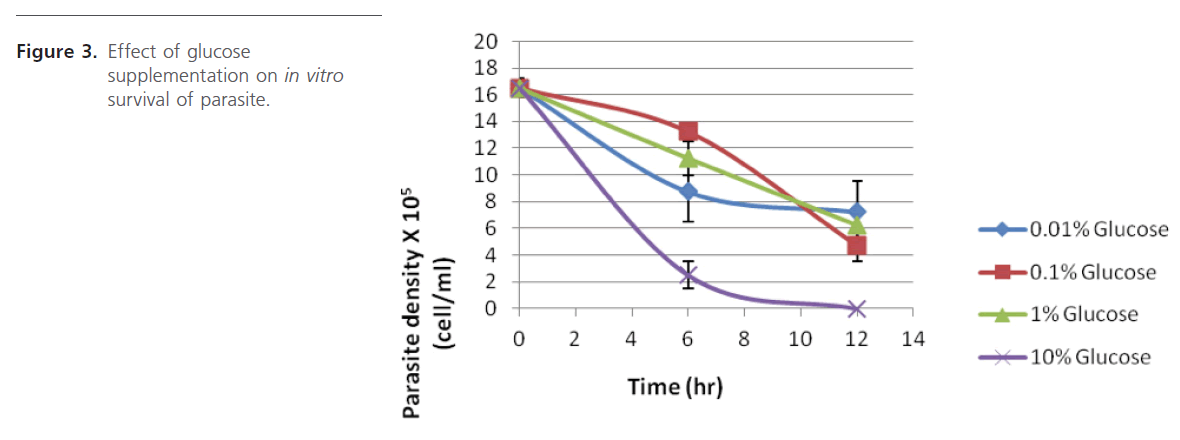
Figure 3: Effect of glucose supplementation on in vitro survival of parasite.
Temperature dependence study (Figure 4) gave optimum growth at 27oC. Several culture temperatures have been used by different research workers, from as low as 24 oC up to 37 oC [8,10,12,22,23]. Study of pH-dependence (Figure 5) indicated possibility of the parasite surviving well in a medium with pH range of 7.0 – 8.0. Higher parasite densities were observed between 7.8 – 8.0, where the medium was buffered with Tris. This may also indicate the superiority of Tris over Phosphate when used to buffer in vitro cultivation processes.
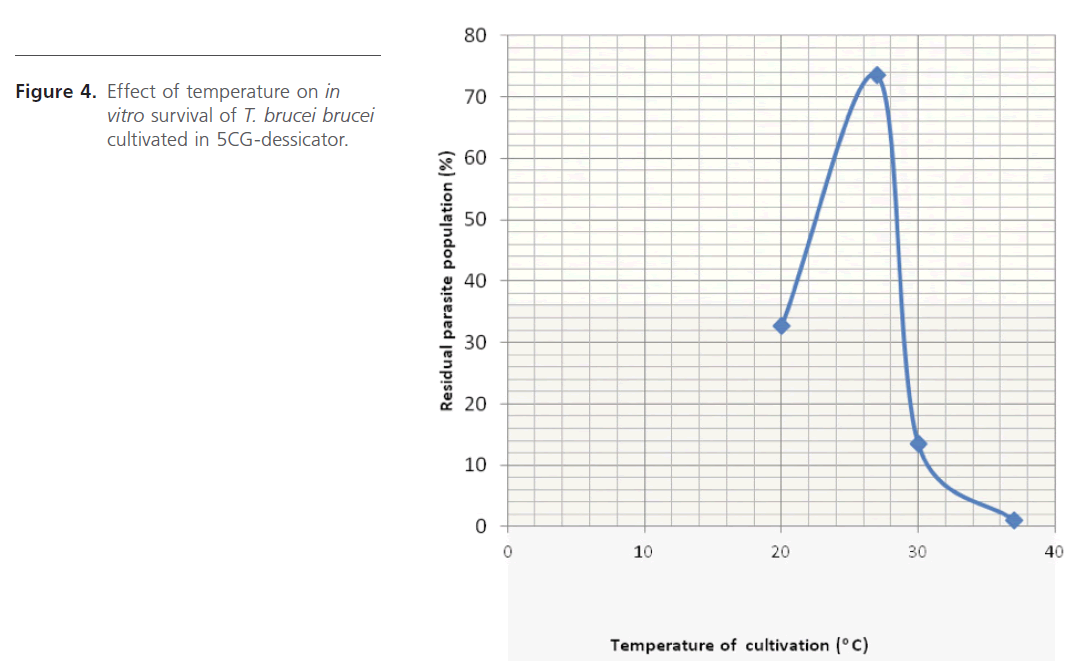
Figure 4: Effect of temperature on in vitro survival of T. brucei brucei cultivated in 5CG-dessicator.
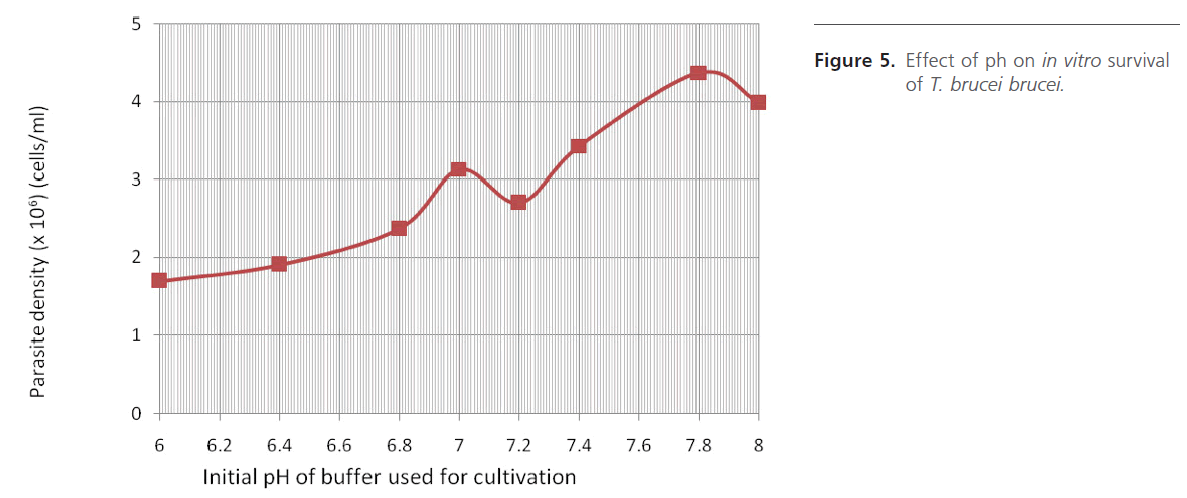
Figure 5: Effect of ph on in vitro survival of T. brucei brucei.
Various media/solutions were also screened for their ability to support parasite in vitro. The study revealed that the parasites cultivated in laboratory-prepared Eagle’s minimum essential amino acid medium survived best (Figure 6). Supplementing this medium with heat-inactivated horse serum even gave better result (Figure 7) which does not seem to vary much when either 10%, 20% or 50% serum was used. Initiation of culture with different parasite densities, prepared by dilution suggests that it is safe to initiate culture with parasite density ranging from 4.5 x106 to 1.7 x 107 cell/ml in order not to have number of parasites too few or too many on the haemocytometer counting field which will require further dilution, a practice that should be avoided.
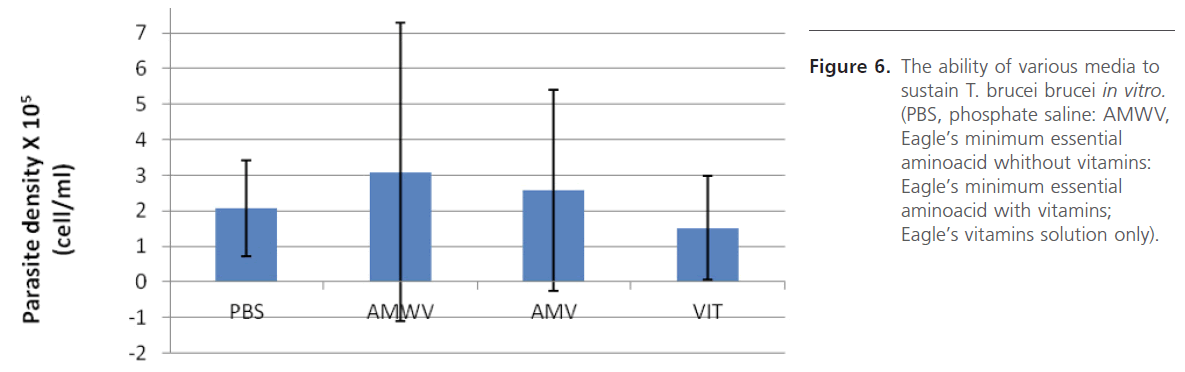
Figure 6: The ability of various media to sustain T. brucei brucei in vitro. (PBS, phosphate saline: AMWV, Eagle’s minimum essential aminoacid whithout vitamins: Eagle’s minimum essential aminoacid with vitamins; Eagle’s vitamins solution only).
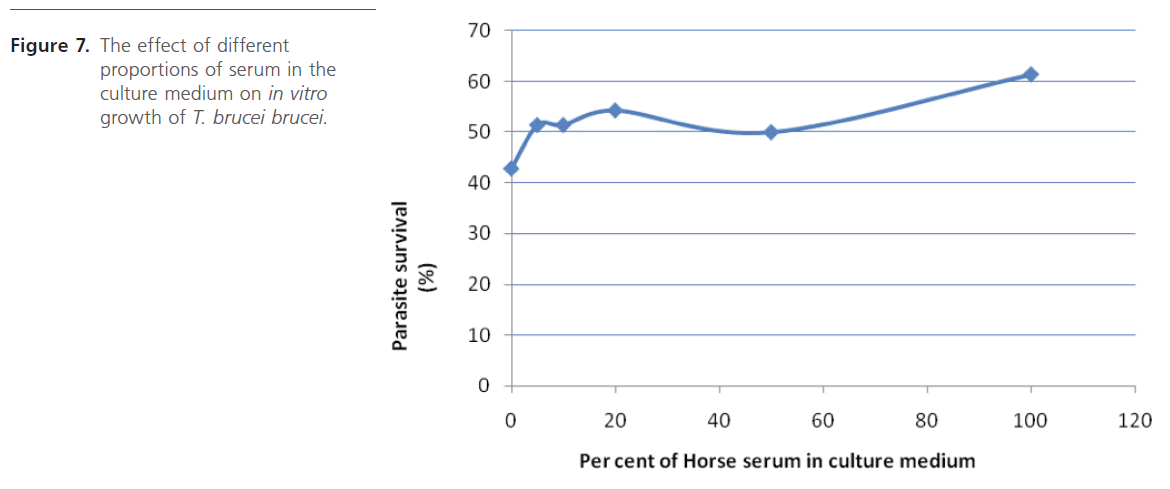
Figure 7: The effect of different proportions of serum in the culture medium on in vitro growth of T. brucei brucei.
Finally, preliminary subculturing experiment (Table 2) shows the possibility of further subculturing already cultured population in microtiter plate using the presented setup. More studies, however, are required to optimize this system. Trying other media with this technique are hereby recommended.
Table 2. Subculturing result of 24 hrs – cultured Trypanosoma brucei brucei in 96-well microtiter plate.
It is significant to note that the pH study was actually carried out in a 96 wells microtiter, which means that this culture approach could be handy when the need for in vitro screening of agents suspected to have antitrypanosomal potentials are used. We are already using this “5CG-desiccator” together with EC50 method of Huber and Koella [24] to determine the IC50 values of various plant extracts with known in vivo trypanocidal activities, a step which will no doubt provide a standard comparative assessment for trypanocidal efficacy of local plants. Additionally, the system does not involve the use of complex equipment such as CO2 incubator and so not quite cumbersome, therefore, could easily be transported and used in areas whose ambient temperatures do not exceed 30oC. This setup, hence, may be very useful to scientists who are involved in field researches.
146
References
- Vickerman, K. (1978) Antigenic variation in trypanosomes. Nature 273: 613-617.
- Onyeyilli, P.A. and Egwu, G.O. (1995) Chemotherapy of African Trypanosomiasis: a historical perspective. ProtozoolAbst 19(5): 229- 241.
- Smith, D.H, Pepin, J, and Stich, A.H. (1998) Human African trypanosomiasis: an emerging public health crisis. Br Med Bul 54(2): 341-55.
- Nok, A.J. (2005) Effective measures for controlling trypanosomiasis. Expert OpinPharmacother 6(15): 2645-2653.
- Gutteridge, W.E. (1985). Existing chemotherapy and its limitations. Br Med Bul 41(2): 162-168.
- Atawodi, S.E., Bulus, T., Ibrahim, S., Ameh, D.A., Nok, A.J., et al. (2003) In vitro trypanocidal effect of methanolic extracts of some Nigerian Savannah plants. Afr J Biotechnol 2(9): 317-321.
- Brun, R. and Balmer, O. (2006). New developments in human African Trypanosomiasis.CurrOpin Infect Dis 19(5): 415-20.
- Hirumi, H., Doyle, J.J. and Hirumi, K. (1977) African trypanosomes: cultivation of animal-infective Trypanosome brucei in vitro. Science 196: 992-994.
- Wink, M. (1979) Trypanosomatheileri: In vitro cultivation in tsetse fly and vertebrate cell culture systems. Int J Parasitol 9: 585-589.
- Baltz, T., Baltz, D., Giround, C.H. and Crockett, J. (1985) Cultivation in a semi-defined medium of animal infective forms of Trypanosomabrucei, T. equiperdum, T. evansi, T. rhodesiense. EMBO J 4(5): 1273- 1277.
- Hirumi, H. and Hirumi, K. (1989). Continuous cultivation of Trypanosomabruceibrucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol 75(6): 985-989.
- Hirumi, H. and Hirumi, K. (1990) In vitro cultivation of Trypanosomacongolense blood stream forms in the absence of feeder cell layers. Parasitology 102: 225-236.
- Lanham, S.M. and Godfrey, D.G. (1970) Isolation of salivarian trypanosomes from man and other animals using DEAE-cellulose. ExpParasitol 28: 521-534.
- Herbert, W.J. and Lumsden, W.H. (1976). Trypanasomalbrucei: a rapid “marching method for estimating the host parasitaemia. ExpParasitol 40: 247-431
- Turgeon, M.L. (2007). Clinical laboratory science the basics and routine techniques. Mosby-Elsevier, pp 263-275.
- Jensen, J.B. and Trager, W. (1977). Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle-jar method. J Parasitol 63: 883–886.
- Scheibel, W., Ashton, S.H., and Trager, W. (1979). Plasmodium falciparum: microaerophilic requirements in human red blood cells. ExpParasitol 47: 410–418.
- Eagle, H. (1955). The specific amino acid requirement of a human carcinoma cell (Strain HeLA). J Exp Med 102: 37-48.
- Fairlamb, A.H., Operdoes, F.R., and Borst, P. (1977) New approach to screening drugs for activity against trypanosomes. Nature 265: 270- 271.
- Bossche, H.V. (1978). Chemotherapy of parasitic infections Nature 273(22): 626-10.
- Simpson, A.M., Hughes, D, and Simpson, L. (1985) Trypnosomabrucei: differentiation of in vitro grown blood stream trypomastigotes into Procyclic forms. J Parasitol 32(4): 672-677.
- Pizzolatti, M.G., Koga, A.H., Grisard, E.C., and Steindel, M. (2002) Trypanocidal activity of extract from Brazilian Atlantic Rain Forest plant species. Phytomedicine 9: 422-426.
- Shuaibu, M.N., Kanbara, T.Y., Icjinose, A., Ameh, D.A., and Nok, A.J. (2003) In vitro trypanocidal activity of dibutyltin dichloride and its fatty acid derivatives. Parasitol Res 91: 5-11.
- 24. Huber, W. and Koella, J.C. (1993) A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 55: 257-261















