Keywords
Brain metastasis; Overall survival; Surgery prognostic factors; Complication and comorbidity.
Introduction
Brain metastasis (BM), intrinsic secondary central nervous tumors, represent by far the most common intracranial tumors in adults [1] and up to 40% of cancer patients develop BM in their disease course [2]. Improved diagnostic methods enable earlier and precise detection of BM. These efforts towards improving systemic cancer therapies resulting in extended patient survival, account for increase in BM incidence [3]. Other than in the case of small cell lung cancer (SCLC) and germ cell tumors [4], BM of other tumor entities tend to be chemoresistant. Hence chemotherapy (CT) is more of an adjunct to local treatment modalities. The later comprise tumor resection by open craniotomy, radiosurgery and local or whole brain radiotherapy (RT) [5]. Ample randomized trials evidence the survival benefit and a recurrence rate reduction of surgery and RT [6-8]. Surgery enables both tumor debulking/removal allowing for symptom relief and obtaining tissue for diagnosis [3]. Successful clinical outcome after surgical intervention is very much dependent on adequate patient selection [3].
Patient related factors such as age, performance status, presence of extracranial metastasis and status of primary tumor are named primary determinants of patient outcome [9]. The classification system developed by the Radiation Therapy Oncology Group (RTOG), the recursive partitioning analysis (RPA), groups patients in respect to outcome according to the former named parameters [10]. However, important surgical and anesthesiological risk parameters such as patient comorbidity, nutritional status and inflammation level are not incorporated in this classification system.
The Charlson-comorbidity-index (CCI) is a comorbidity metric, which provides a simple readily applicable and valid method for estimating risk of death from comorbid disease. It also takes into account the number and the seriousness of comorbid disease [11]. Our recently published study showed that in addition to the already established prognostic parameters (age and Karnofsyk performance score (KPS)) for Glioblastoma patient outcome, the CCI significantly impacts outcome and may be employed for preoperative patient stratification [12].
Nutritional depletion has previously been reported as showing an association with poor outcome and increased risk of complications, particularly postoperative infections [13]. Moreover, recent reports in melanoma metastasis state inflammatory chemokines to be gaining rapid momentum in the biomarker discovery domain. Hence, aiding melanoma prognosis and high-risk patient stratification [14].
Our retrospective review of consecutive BM patients treated in a neurosurgical department, aimed at identifying additional preoperative stratification parameters assessing nutrition, inflammation and comorbidity and evaluating impact on patient survival, perioperative morbidity and mortality.
Methods
Patient selection
Consecutive adult patients treated for BM at a single neurosurgical department over an 18-months period were retrospectively reviewed. Tumor diagnosis was confirmed by histopathological analysis conducted by a certified pathologist. Patients with incomplete medical records deficient of data on clinical presentation, pre- and postoperative imaging were excluded aiming at generating a uniform patient population with similar diagnostic and treatment strategies. One hundred patients met the inclusion criteria and composed the final study group. Institutional review board approval was available for all aspects of the study and all patients consented to have their medical records assessed.
Patient, tumor and clinical characteristics
Information recorded included treatment of the primary tumor, surgical resection, Biopsy (open/stereotactic), peri- and postoperative course comprising adjuvant treatment after BM surgery and follow-up data. Patient demographics were classified as age, gender and date of surgery. The date of BM imaging (contrast enhanced magnetic resonance images (MRIs) obtained preoperatively) marked the date of BM diagnosis. Chart review and data assessment was independent and blinded to outcome. Preoperative comorbidity was indexed by the CCI as described elsewhere [11,15] and further dichotomized as being >2 or 70% or 10]. Status of extracranial metastatic disease (stable, progressive or simultaneous diagnosis) was noted. Image assessment allowed the computing of the number of BM and the size measurement. The diameter of the largest lesion was dichotomized as 3 cm. Lobe localization of the BM (supra-/infratentorial, left/ right/midline) was also recorded. All patients were measured for body weight (kg) and height (meter). The Body mass index (BMI) was obtained from Quetelet’s index (weight in kilograms divided by the square of height in meters). Preoperative serum C-reactive protein (CRP) levels were measured for all patients and dichotomized as normal/elevated 0.5 mg/L respectively, according to our institutional laboratory criteria.
Outcome
Complications were divided into 3 groups; neurological, regional/surgical and systemic/medical as suggested by previous publications, which assessed patients undergoing craniotomy [16].
Patients were followed up clinically and by MRI every 3 months. Survival data were collected from patients’ visits to the clinic or during the phone interview with patients and/or their relatives or physicians. Death data was updated through to May 2015. Patients who were still alive at last follow-up were considered as a censored event in analysis. The primary study endpoint of overall survival (OS) was calculated from the date of BM- or primary tumor imaging. In cases in which death could not be confirmed by any means, the patients were classified as lost to follow-up at the time of their last clinic visit. Furthermore, the 30- day mortality, in-hospital mortality and complication rates were recorded.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, Illinois, USA). Descriptive data was presented as means ± SDs for parametric data and medians with IORs for nonparametric data. Group differences were assessed via Fisher exact test /Mann-Whitney U-test for categorical variables and the Student t-test for continuous variables as appropriate. P values <0.05 was considered statistically significant. Factors independently associated with occurrence of complications were assessed by univariate analysis. The Kaplan-Meier-method and Log-rank analysis was used to assess the impact of factors on outcome. Independent variables proving significant served as covariates for multivariate logistic regression analysis.
Results
Perioperative characteristics
Table 1 gives a summary of patient demographics, clinical data and tumor characteristics of the 100 patients, which made up the final study cohort (43% female and 57% male). Median age at first diagnosis of BM was 64 years (range 45-82). Majority of patients 91% presented with a good preoperative functional status showing a KPS>70%. Respectively, only 9 patients were classed as RPA III, most were of classes II (69%) and I (22%). The range of recorded patient comorbidity was 1-6 with an even distribution of patients with a score <2 (49%) and >2 (51%). Median BMI was 24.7 (14.7-39.9). Five patients had BMI of 20 or less. Half of the study population presented with ideal body weight (BMI 20-25). 29% of patients were over weight (BMI 25-30) and 16% were obese (BMI<30). The mean serum CRP level was 2 ± 0.31 mg/L and elevated levels were evident in 43% of patients. Preclinical symptoms were apparent in 85 cases, hereof cognitive defects were most frequent with 31% (22 cases) and least often observed were intracranial pressure symptoms and speech deficits with 13% (9 cases) each.
| Preoperative Characteristics |
|
|
| Sex |
|
n/100 =% |
| |
Female |
43 |
| |
Male |
57 |
| Age at BM diagnosis |
|
Median (range) years |
| |
Total study group |
64 (45-82) |
| Body mass index (BMI) |
|
|
| |
Median (range) |
24.7 (14.7-39.9) |
| |
|
n/100=% |
| |
<20 |
5 |
| |
20-25 |
50 |
| |
25-30 |
29 |
| |
>30 |
16 |
| C-reactive protein (CRP) |
|
|
| |
Mean +-SD |
2 +-0.31 |
| |
Elevated (yes/no) |
43/56 |
| Prognostic scores at diagnosis of BM |
|
n/100 =% |
| KPS |
|
|
| |
<70% |
9 |
| |
>70% |
91 |
| CCI |
|
|
| |
<2 |
49 |
| |
>2 |
51 |
| RPA class |
|
|
| |
I |
22 |
| |
II |
69 |
| |
III |
9 |
| Presenting signs and symptoms 30d before BM diagnostis |
|
n/100 =% |
| |
Asymptomatic |
15 |
| |
Symptomatic |
85 |
| Nature of symptoms |
|
n/85 (%) |
| |
Headache |
20 (14) |
| |
Cognitive deficit |
31 (22) |
| |
Seizure |
19 (13) |
| |
Speech deficit |
13 (9) |
| |
Paresis |
29 (20) |
| |
Cranial nerve palsy |
19 (13) |
| |
Intracranial pressure signs |
13 (9) |
| Status of primary tumor at diagnosis of BM |
|
n/100 =% |
| |
Stable disease |
40 |
| |
Progressive disease |
14 |
| |
Synchronous diagnosis of primary tumor and BM |
46 |
| 1st diagnosis of °tumor via BM |
|
|
| |
Yes |
40 |
| |
No |
60 |
| Number of BM at diagnosis of BM |
|
|
| |
1 |
70 |
| |
>1 |
30 |
| Extracranial metastases at BM diagnosis |
|
|
| |
Solitary (BM only site/no extra cranial metastases) |
70 |
| Organ |
Liver |
2 |
| |
Lung |
5 |
| |
Bone |
1 |
| |
> 1 organ |
12 |
| |
Others |
5 |
| |
Unknown |
4 |
| BM Localization |
|
|
| |
Left lobe |
51 |
| |
Right lobe |
37 |
| |
Both |
12 |
| |
Supratentorial |
69 |
| |
Infratentorial |
31 |
| BM Size |
|
|
| |
<3cm |
40 |
| |
≥3 cm |
60 |
| Histology of BM |
|
|
| |
Lung Cancer (NSCLC/SCLC) |
54 (35/19) |
| |
Breast Cancer |
9 |
| |
Melanoma |
13 |
| |
Renal cell carcinoma |
6 |
| |
Prostate Cancer |
2 |
| |
Colorectal Cancer |
10 |
| |
Stomach Cancer |
3 |
| |
Esophagus Cancer |
2 |
| |
Cancer of unknown primary |
1 |
| Therapy |
|
|
| Therapy primary tumor before BM diagnostic |
|
|
| |
CT |
8 |
| |
CT+RT |
11 |
| |
Surgery |
31 |
| |
None |
44 |
| |
Surgery + (CT/CT+RT) |
6 |
| |
Brain RT |
5 |
| Surgical therapy of BM |
|
|
| |
Resection |
95 |
| |
No. of BM resected |
|
| |
1 |
87 |
| |
2 |
8 |
| |
Biopsy (open/stereotactic) |
5 (3/2) |
| Adjuvant treatment for BM |
|
|
| |
CT |
14 |
| |
LBRT |
17 |
| |
WBRT |
9 |
| |
LBRT+WBRT |
8 |
| |
CT+LBRT |
8 |
| |
CT+WBRT |
16 |
| |
CT+LBRT+WBRT |
4 |
| |
Best supportive care |
13 |
| |
Sonstige |
4 |
| |
Unknown |
7 |
| Outcome |
|
|
| Perioperative 30d morbidity and mortality rates |
|
n/100 =% |
| |
In-hospital mortality |
4 |
| |
Mortality |
17 |
| |
Morbidity |
29 |
| |
Reoperation |
4 |
| |
Readmission |
5 |
| Complications |
Total No. cases |
41 |
| Subgroups |
|
n/41 (%) |
| |
Neurological |
11 (27) |
| |
Surgical |
9 (22) |
| |
Medical |
21 (51) |
| Recurrent BM rate |
Total No. cases |
8 |
| |
Local |
5 |
| |
Distant |
3 |
| Re-Resection of recurrent BM |
|
5 |
| Overall Survival |
|
n/100 =% |
| |
Dead at last FU |
48 |
| |
Alive at last FU |
39 |
| |
Lost to FU |
13 |
| |
|
Median (range) months |
| |
OS from diagnosis of primary tumor |
28 (13-43) |
| |
OS from diagnosis of BM |
10 (3.5-16.5) |
Table 1: Summary of case characteristics and outcome.
One third of tumors were located supratentorialy, 51% were sited in the left lobe. Also, 60% of tumors were larger than 3 cm in diameter. 95 tumors were resected by open craniotomy, 3 addressed by open biopsy and consequently in two cases stereotactic biopsy was performed for diagnostic purposes. In the resected cases mostly one lesion was resected 87 out of 95 cases. Status of extracranial disease was stable in 40% and progressive in 14 cases. Simultaneous diagnoses of primary tumor and BM was in 46 cases of which 40 manifested primarily via BM diagnosis. Forty-four patients had not received any therapy of the primary tumor at BM diagnosis. Lung cancer and melanoma rated most frequently with 54% and 13% respectively. Radiotherapy of the brain pre BM resection was recorded in 5 patients. Detailed information on adjuvant therapeutic regimes employed after BM resection/biopsy showed that CT and RT both (local and whole brain RT) was employed in 76 patients. 13 patients received best supportive care.
Outcome and risk factor analysis
Data on survival was attainable for 87 patients as depicted in Table 1. Median OS from primary tumor diagnosis was 28 months (range 13-43) Figure 1a. The median survival after BM diagnosis was 10 months (range 3.5-16.5). 48% of patients were dead at last follow up. 30-day morbidity rate was 29% (41 cases). The overall 30-day mortality rate was 17% of these patients, 4% died as a result of in-hospital perioperative complications. Medical complications outnumbered with 51%, whereas surgical and neurological complications occurred with similar rates of 22% and 27% respectively. 4 patients had to be re-operated for surgical complications (3 cases of subdural bleeding and one wound infection). 8 patients showed recurrent tumors 5 local and 3 distant metastasis. We re-operated on the 5 local cases. Assessment of factors impacting survival showed that occurrence of complications (p=0.001), age (p=0.024), solitary BM (p=0.007) significantly impacted low survival Figure 1b. Consequent grouping of patients by RPA class showed that a higher RPA class (p<0.0001) significantly impacted poor survival Figure 1b. Multivariate logistic regression analysis showed high RPA class (p<0.001, OR 3.52, 95% CI 1.80-6.90) and complication occurrence (p=0.05, OR 2.36, 95% CI 1.30-4.28) to independently impact poor survival.
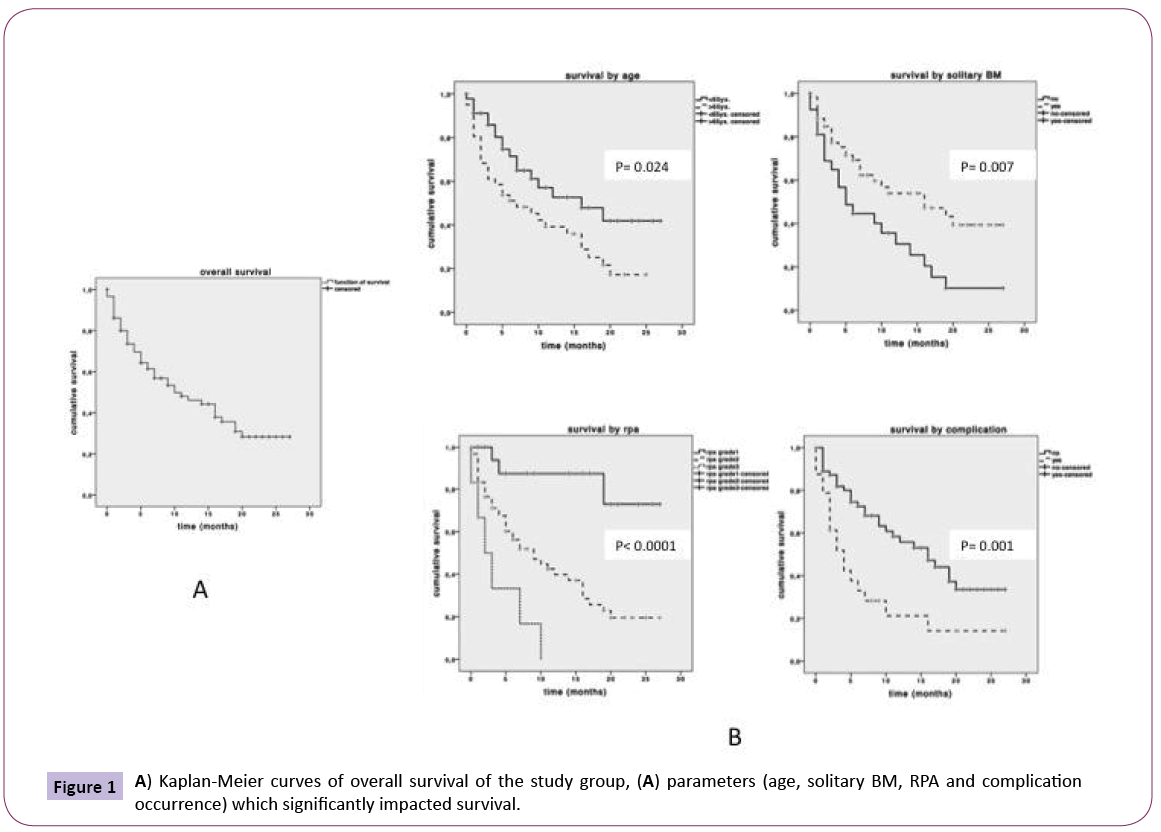
Figure 1: A) Kaplan-Meier curves of overall survival of the study group, (A) parameters (age, solitary BM, RPA and complication occurrence) which significantly impacted survival.
Univariate analysis of factors risking for complication occurrence showed that none of the assessed parameters including BMI, CRP and CCI proved significant impact.
Discussion
Mortality rates
Our in-hospital mortality rate of 4% relates to published results [7,9] where a 30-day postoperative and postradiotherapy mortality rate of 4% is described. The 17% overall 30-day mortality rate we observed also comprised patients who died due to the underlying disease. This is depicted by the 13% of patients receiving best supportive care. Our results strengthen other author’s opinion of craniotomy not being riskier than whole brain RT in the short term [8]. Further, advantages of surgery over RT are that the later has long-term neurotoxicity, is not eligible for large lesions and does not yield in mass reduction [6].
Similar to our study, reports in literature of mixed tumor histology, state a median survival time of 11 months (range 6 to 16). In our study, 39% of patients were alive at last follow up, the 1 year OS was 16% with a mean OS of 28 months. Pachtell et al state in their Randomized trail, that the survival after BM surgery is of more relevance than OS. The time of death from BM diagnosis was 10 months in our study, this compares to mono histological studies where survival times ranged from 5 months for melanoma metastasis to 14 months for NSCLC. Opposing to literature reports where breast cancer metastasis is reported as the second frequent tumor entity to lung cancer [5], we had melanoma second to non SCLC and SCLC Table 1. This discrepancy may be attributed to the fact that a major skin cancer center referred an unusually high number of patients to our institution.
Morbidity and risk factors impacting patient outcome
The complication rate in our study of 29% is higher than literature reports where the general 30-day morbidity rate is reported between 8%-10% [7,17,18]. Mere numerical comparisons here are not adequate as some studies report on the number of patients rather than the number of events. We recorded each complication event observed irrespective of the severity as opposed to other literature reports [6]. Gempt et al reported of temporary and permanent neurological deterioration to be significantly associated with ischemic lesions, which tended to occur more often in patients who had had radiation therapy [17]. In our study, 27% of complications were neurological; however there was no association to presurgical radiation therapy. This had been performed only in 5% of our patients. These differences may be attributed to the fact that our study focused only on permanently evident complications and we did not make correlations to imaging data.
Our study group showed a low recurrence rate of 8%. In literature recurrence rates between 12.5-40% are reported [18-20]. This wide range was not related to the complication rate but to the resection method employed; en bloc versus piecemeal [9] and the adjuvant therapy used to treat the BM [20]. Although we did not specifically report on the surgical resection technique and extent of resection, the general low recurrence rate in our cohort allows the assumption of good resection extents. Generally, it is reported that reoperation can improve patients´ quality of life if symptom relief is attained with an expected 2 year and 5 year survival rate of 26% and 17% respectively [18]. In our review, reoperation was performed in 5 out of the 8 patients. Specifically, in patients who experienced local recurrence. The low risk of reoperation is emphasized by the fact that none of these patients experienced a 30-day mortality or morbidity. Hence, patients with recurrent BM tumor are worth considering for repeat surgery.
The highly significant association between RPA class and survival, confirmed by the significant impact of age and solitary metastasis reflects the expected confounding effect Figure 1. This is because the RPA classification encompasses the factors of age and extent of metastasis. Our findings therefore confirm previous reports of RPA being a good tool for preoperative patient stratification [21,22]. Aside RPA class our results further proved occurrence of complications to independently impact on survival at the multivariate level. In order to help stratify for patients at risk for developing a complication we further analyzed factors for association with complication occurrence. Interestingly none of the parameters we assessed had a significant relation to complication occurrence.
Many studies including our own reports describe the CCI to be a holistic metric for comorbidity assessment, capable of predicting the 10-year mortality of patients. However, in this review, we did not observe an outcome relation between the CCI and BM patients’ mortality and morbidity. This may be because BM patient death was more a course of cancer disease rather than a result of comorbidity.
More often than not craniotomy for patients is delayed with the aim of improving the presurgical status and reducing perioperative mortality and morbidity risk. In some cases, even patients are ruled out for resection arguing on the high number of comorbidities. Considering our findings that for BM patient’s neither poor morbidity nor mortality correlated to CCI, BMI and CRP levels, such decisions may not be justified. They may not only impair therapy but also deprive patients of adequate local tumor therapy. Hence, when patients have a good RPA score and other factors including tumor location and liability for anesthesia are favorable, these patients should not be ruled out for tumor surgery. The fact that only the complication rate and RPA class impacted survival at the multivariate level and other factors including CCI, BMI and CRP levels neither impacted complication nor survival rate may reflect that, these measures do not adequately assess for BM outcome. Hence, when assessed preoperatively it should not be a reason to delay patients´ surgery or onset of further adjuvant treatment. In terms of CCI, one might argue that a score developed for longitudinal studies is not suitable for BM patients with short survival data. Therefore, to comprehensively assess complication risk factors for BM more extensive randomized surveys comparing other comorbidity scores have to be conducted.
Study strengths and limitations
The relatively small study population of 100 patients and the retrospective study design limits our report. However, the fact that our mortality and morbidity rates relate to literature reports [7,9] shows a degree of data accuracy and consistency allowing us to legitimately make comparisons and draw conclusions.
Conclusion
Essentially, our analyses confirm the value of RPA-derived prognostic classes for survival and proved complication occurrence to be a further significant impact factor. This emphasizes the importance of reducing perioperative complication rates. CCI, BMI and CRP as measures of nutrition, inflammation and comorbidity respectively, not significantly impacting on mortality or morbidity may be due to the fact that these factors are not eligible preoperative selection parameters. Consequently, patient selection for surgery should not be based on these factors.
Acknowledgements
We thank Sandra Birkner and Johannes Vogel for assistance in data acquisition.
Funding
Authors received no funding for the study.
Competing Interests
The authors have no conflicts of interest to declare.
6683
References
- Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5-14.
- Sul J, Posner JB(2007) Brain metastases: epidemiology and pathophysiology. Cancer Treat Res136:1-21.
- Al-Shamy G, Sawaya R (2009) Management of brain metastases: the indispensable role of surgery. J Neurooncol 92:275-82.
- Mahalati K, Bilen CY, Ozen H, Aki FT, Kendi S (1999) The management of brain metastasis in nonseminomatous germ cell tumours. BJU Int 83:457-461.
- Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, et al. (2012) Brain metastases: pathobiology and emerging targeted therapies. ActaNeuropathol123:205-222.
- Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, et al. (2008) Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol87:299-307.
- Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, et al. (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494-500.
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, et al. (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583-590.
- Patel AJ, Suki D, Hatiboglu MA, Rao VY, Fox BD, et al. (2015) Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg1-12.
- Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, et al. (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J RadiatOncolBiolPhys 37:745-751.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987)A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373-383.
- Ening G, Osterheld F, Capper D, Schmieder K, Brenke C (2015) Charlson comorbidity index: an additional prognostic parameter for preoperative glioblastoma patient stratification. J Cancer Res ClinOncol 141:1131-1137.
- Dempsey DT, Mullen JL, Buzby GP (1988) The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J ClinNutr47:352-356.
- Neagu M, Constantin C, Longo C (2015)Chemokines in the melanoma metastasis biomarkers portrait. J Immunoassay Immunochem 36:559-566.
- Balducci M, Fiorentino A, De Bonis P, Chiesa S, Manfrida S, et al. (2012) Impact of age and co-morbidities in patients with newly diagnosed glioblastoma: a pooled data analysis of three prospective mono-institutional phase II studies. Med Oncol 29:3478-3483.
- Chang SM, Parney IF, McDermott M, Barker FG, Schmidt MH, et al. (2003) Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg 98:1175-1181.
- Gempt J, Gerhardt J, Toth V, Huttinger S, Ryang YM, et al. (2013) Postoperative ischemic changes following brain metastasis resection as measured by diffusion-weighted magnetic resonance imaging. J Neurosurg 119:1395-1400.
- Bindal RK, Sawaya R, Leavens ME, Hess KR, Taylor SH (1995) Reoperation for recurrent metastatic brain tumors. J Neurosurg 83:600-604.
- Patel AJ, Suki D, Hatiboglu MA, Abouassi H, Shi W, et al. (2010) Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg 113:181-189.
- Nieder C, Astner ST, Grosu AL, Andratschke NH, Molls M (2007) The role of postoperative radiotherapy after resection of a single brain metastasis. Combined analysis of 643 patients. StrahlentherOnkol 183:576-580.
- Tendulkar RD, Liu SW, Barnett GH, Vogelbaum MA, Toms SA, et al. (2006) RPA classification has prognostic significance for surgically resected single brain metastasis. Int J RadiatOncolBiolPhys 66:810-817.
- Zhang Q, Chen J, Yu X, Ma J, Cai G, et al. (2013) Systemic treatment after whole-brain radiotherapy may improve survival in RPA class II/III breast cancer patients with brain metastasis. J Neurooncol 114:181-189.






