Keywords
Public health, Aflatoxin M1, Human breast milk, Immunoaffinity column, Nigeria, Women.
Introduction
The contamination of food with aflatoxin is a global one; however, it is more serious in tropical countries of the world where relative humidity is high and temperatures conducive for the growth and production of aflatoxin by moulds. Aflatoxin (AFs) are potent carcinogens that are produced as secondary metabolites of strains of Aspergillus parasiticus and Aspergillus flavus that grow on important food crops such as groundnuts, maize and other oilseeds [1-3].
Breast milk is considered to be the best food for the infant [4-5] and the importance of breast feeding for normal growth and development has become increasingly recognized. However, in addition to its nutritionally and immunologically beneficial components, human milk may contain trace amounts of a wide range of contaminants. The occurrence of mycotoxins in human, animal and milk formula is probably one of the most serious public health concerns since milk is an important food for adults and unique nutrient for infants [6-7]. The latter are extremely vulnerable because their diet is much less varied and depends on mothers’ dietary habits. It has been reported that in human milk variable amount of mycotoxins ingested by the mother can be accumulated in an intact metabolized form [8-10]. Approximately 95% of AFB1 metabolites excreted in milk are in the form of AFM1, though AFM2, AFG1 and AFB1 have been reported. Although AFM1 is considered a detoxification product, there is evidence of a putative AFM1 carcinogen role as reported by Peers, D’Mello and Macdonald, Codex Committee on Food Additives and Contaminants [11-13].
In spite of the recognized need for frequent surveys of food items for mycotoxins, few studies are available on the occurrence of aflatoxins in biological fluids. In addition to being potent carcinogens, aflatoxins may contribute to early child growth retardation and particularly strong associations between aflatoxin and child growth [14-19] have been reported around the weaning stage in Beninese infants [20]. A study conducted in Gambian children reported that secretory IgA in saliva may be reduced by dietary levels of AF [17]. The immune status of Ghanaians adults has been reported to be affected by aflatoxin exposure [21].
Milk is the main nutrient for the first six months of life as has been entrenched in the Nigerian Health Programme. There is the possibility that these children who are the future of the nation are exposed to doses of aflatoxins. Infants are more susceptible to the adverse effects of aflatoxins. It has been stated that African countries should be devoted to monitoring milk and milk products other than those from cows as previously shown [17]. Presently there is lacuna of data on aflatoxin M1levels in human breast milk in Nigeria. Therefore it is important to monitor aflatoxins levels in human breast milk in Nigeria since it is a risk of serious health hazard to mother, foetus and infants.
Material and Methods
Study population and sample collection
Breast milk samples were collected from 120 Nigerian women that agreed to participate in the study (participation rate 98%) during the month of June, 2008. These women were from two hospitals in two cities in South Western Nigeria (Ibadan and Abeokuta). This is a tropical rain forest zone with high humidity and temperatures which promote mould growth and aflatoxin production. The people are famous for consumption of foods such as maize, yam chips, groundnuts, groundnut oil, melon, cassava-based foods, beans, rice, and wheat bread. Breast milk samples were collected (5ml) for aflatoxin determination into universal bottles and stored at 4°C prior to extraction within 24hours.
Ethical considerations
The study was approved by the Ethics committee of the Ministry of Health, Oyo state of Nigeria. All subjects were made aware of the content of the study and if they agreed to participate breast milks were collected from willing mothers manually.
Collection of dietary, socioeconomic, demographic and clinical data
The intake of dietary sources of aflatoxins was assessed by asking women about their diet and eating habits focusing mainly on foods more likely to be sources of aflatoxins [3,22]. The focused foods were cereals, maize, yam chips, groundnut oil, melon and nuts used for soups, beverages (bournvita, ovaltine, and chocovita) containing cocoa, dried fish, dried meat and milk.
Validation and Reproducibility of measurement
Validation of measurement was done by using Hipp Baby formula bought from supermarket in Munich, Germany. Seventy grams of powdered milk were weighed into a 1-litre beaker and 450ml of Millipore water added. The mixture was stirred vigorously with a stirrer for 5 minutes. The temperature of the milk was raised to 50° C to ensure proper dispersion of milk fat. There after milk temperatures were lowered to room temperature. Four centrifuge tubes containing milk were put in the cold room (4°C) for 15min. The solidified fat were scooped off and the milk samples filtered using ashless filter circles (MN 640W, diameter 150mn) (Macherey-Nagel, Germany). Seventy milliliters of defatted Hipp milk were measured into 100ml of volumetric flask. Three flasks containing Hipp defatted milk was spiked with 25ng/ml AFM1 and two samples served as controls. AFM1 was extracted as described below. The average recovery rates were calculated.
Breast milk aflatoxin extraction
Breast milk was collected into universal plastic bottles by selfexpression. The samples were collected into iced cooler and stored at 40 C within 24hours before extraction. Extraction was done following the method of Lamplugh [23].
Briefly 1-ml of hexane was added to each sample and centrifuged (3000 x g, 5min) and the hexane layer removed. This was repeated twice for each sample. Thereafter, 1-ml of chloroform was added to the defatted milk and centrifuged (4,000 x g, 10min). The chloroform layer was collected into plastic bottles. This was repeated four times and chloroform layer pooled together. The extracts were kept in a dark cupboard overnight to dry. The dried samples were taken to Germany for High Performance Liquid Chromatography (HPLC) analysis.
Analysis. Chemicals and solvents used were HPLC grade or equivalent. All water used was distilled and for HPLC, passed through a Milli-Q purification system (Millipore, UK) and acetonitrile used was HPLC grade (Merck) .
Immunoaffinity columns (RIDA aflatoxin column, R-BIOPHARM, Darmstadt) were used for clean-up of the sample extracts. The immunoafinity column (IAC) was brought to room temperature, plugged unto Luer-attachment of a vacuum pressure facility. A solvent reservoir column was attached to the top of the IAC and 10 mls of Millipore water were added to dried extract and vortexed before transferring into the reservoir. The sample was allowed to flow through the IAC at a rate of 1ml min-1. Slight vacuum pressure was applied. The column was washed twice with 10 mls each of Millipore water at a flow rate of 1ml min-1. The reservoir was removed and the IAC was dried by applying slight pressure. Aflatoxin M1 was collected into glass bottles previously treated with 2N H2SO4. The solvent used for eluting was 1.25-ml methanol/ acetonitrile (20:30, v/v). The eluate was dried in a water bath at 400C and 80kPa. The dried extract was re-dissolved with 1.25-ml of acetonitrile: water (25:75, v/v). The solution used for determination of aflatoxin M1.
HPLC determination of aflatoxins
The HPLC system consisted of a LDC, Milton Roy, Consta Metric 1 pump and a Lichrosorb RP-18 (Merck Hibar) column (particle size of 5 μm, length, 125 mm, inside diameter, 4mm). The pump pressure was 60MPa. The Injector was automatic type (Rheotype, Gilson Abimed Model 231). The detector had a fluorescence spectrometer (Shimadzu RF 535, gamma excitation, 365 mm, gamma emission, 44nm. The flow rate was 1ml min-1 and the injection volume was 50μl. The mobile phase was water/acetonitrile (75:25).
Standard solutions
Aflatoxins M1 was obtained from Sigma-Aldrich (St. Louis, MO, USA). The commercial stock solution of AFM1 was 1000ng/ml. The spike solution was made by diluting the stock solution 1:40 approximately 25ng/ml using HPLC grade acetonitrile/water. Out of the diluted stock solution, 140 μl were added to 70ml of defatted Hip baby milk.
Calibration curve was prepared by diluting 2μg/l of AFM1 in a 1:500 dilution. The concentrations of standards were prepared to give the following 1.5μg/l, 1.0μg/l and 0.25μg/l. The stock solutions were stored at 40 C when not in use. For recovery experiments, uncontaminated baby formula milk was spiked with AFM1 at 2.5ng/ml.
Statistical analysis
Comparison between means was evaluated by student t-test and analysis of variance. A value of p< 0.05 was considered significant.
Results
Average recoveries for reproducibility measurements ranged from 89 to 103%. The reproducibility of measurement is shown in Table 1. The calibration curves showed good linearity.
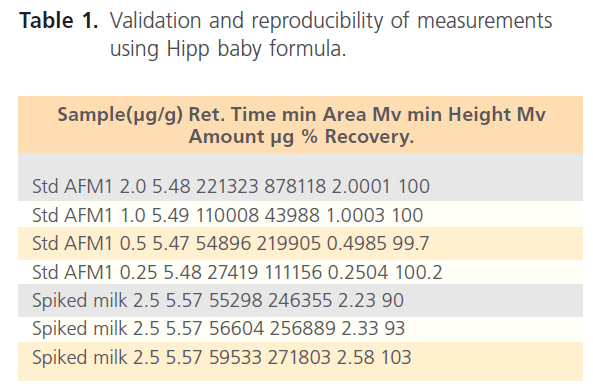
Table 1. Validation and reproducibility of measurements using Hipp baby formula.
AFM1 was found in 17 samples (14.1%) as shown in Table 2. The contamination level of AFM1 ranged from 2-187 ng/ml. HPLC scans of standard aflatoxin M1 are shown together with aflatoxins in breast milk samples of women (figures 1, 2 and 3). T-test using SSPP version 14.0 at 95% confidence interval of difference gave 0.332 AFM1. Data obtained from subjects indicated all consumed basic diets such as maize, yam, cassava, groundnut, melon and bread. Consumption of basic food staples might have contributed to levels of aflatoxins detected in subjects, however individual ability in relation to state of health to effectively detoxify these toxins may have played significant role in the number of positive women.
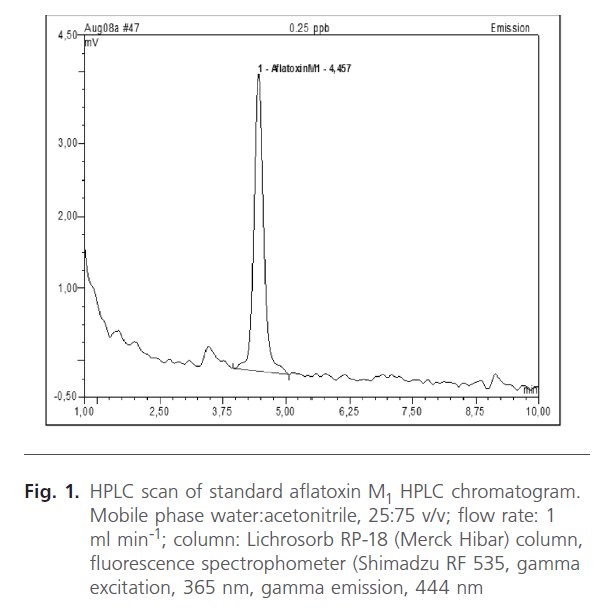
Figure 1. HPLC scan of standard aflatoxin M1 HPLC chromatogram. Mobile phase water:acetonitrile, 25:75 v/v; flow rate: 1 ml min-1; column: Lichrosorb RP-18 (Merck Hibar) column, fluorescence spectrophometer (Shimadzu RF 535, gamma excitation, 365 nm, gamma emission, 444 nm

Figure 2. HPLC chromatogram of quantified aflatoxin M1 in sample 27B equivalent to 187ng/ml. Mobile phase water:acetonitrile, 25:75 v/v; flow rate: 1 ml min-1; column: Lichrosorb RP-18 (Merck Hibar) column, fluorescence spectrophometer (Shimadzu RF 535, gamma excitation, 365 nm, gamma emission, 444 nm
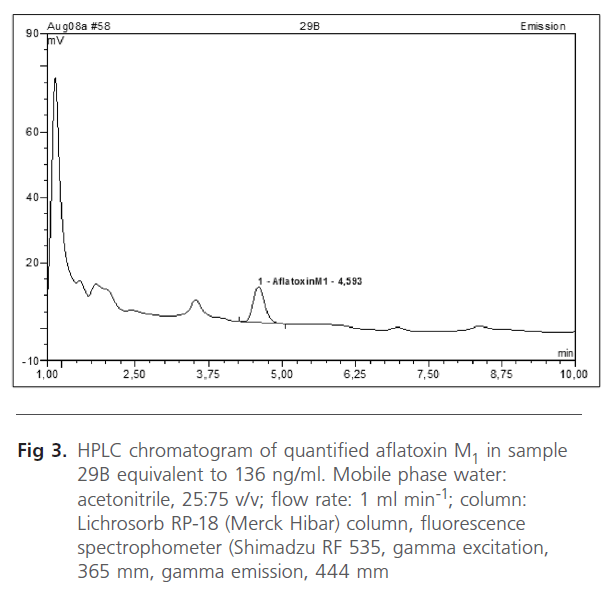
Figure 3. HPLC chromatogram of quantified aflatoxin M1 in sample 29B equivalent to 136 ng/ml. Mobile phase water: acetonitrile, 25:75 v/v; flow rate: 1 ml min-1; column: Lichrosorb RP-18 (Merck Hibar) column, fluorescence spectrophometer (Shimadzu RF 535, gamma excitation, 365 mm, gamma emission, 444 mm
Discussion
Children are highly susceptible population group when exposed to environmental toxicants for a variety of reasons, including lower detoxification capacity, rapid growth, and higher intakes of air, food and water per kg body weight [24]. Therefore, early childhood exposure to carcinogenic aflatoxins may be critical determinants of immediate and later health effects. AFM1 in breast milk is a marker of dietary exposure of mother to AFB1 and consequently aflatoxin exposure to breast-feeding infants as previously shown [8]. In this study, 14.2% of mothers were positive for AFM1 in breast milk although some values were on the high side. Three mothers had AFM1 values above the stipulated EU level of 50 ng/ml in baby food. In this study, the mean aflatoxin in milk was 44 ng/ml for AFM1.
The results in this study show that some lactating women in Ibadan and Abeokuta cities in Nigeria are exposed to low to moderate levels of aflatoxin M1. Daily intake of aflatoxin M1 by the infants will vary depending on the diet of the mother who possesses it or via the breast milk.
Though aflatoxin or its metabolites may be stored in fat reserves the levels and contribution, if any, to breast milk levels at given stages of lactation are not known. Secondly, the fat content and total milk volume vary within a feed and between feeds, and this may impact on the levels of aflatoxins observed. Thirdly, the average volume an infant will consume a day will vary. Despite these variations one can make some estimate of aflatoxin M1 intake in positive mothers with detectable AFM1. Assuming an average daily milk volume of 500ml, the average intake of AFM1 by the infant can be estimated to be 22.0 μg/day and the range will be 1.0 -93.5 μg/day. This value is slightly moderate compared to low values obtained by other scientists somewhere around the globe as previously cited. This difference might be due to the fact that samples of breast milk were collected from women in the tropics where aflatoxin contamination of food is highest in the whole world. Some of these children may also be introduced to wean foods that are likely to be contaminated [24]. Thus the aflatoxin burden in children is increased
In Nigeria, the sources of aflatoxin exposure are well-defined as previously shown [3]. A major contributing factor is poor post harvest management of the harvested crops. In small farmer communities, the crops harvested are usually left to dry over plastic or synthetic sheets, which may promote Aspergillus growth and toxin production as previously as previously shown [15]. Most of the crops are consumed locally by either the household or close communities. Worldwide, two of the major sources of aflatoxin exposure are groundnuts and corn. These two crops are mainly consumed in Nigeria in several forms as boiled corn, roasted corn with groundnut and groundnut oil.
In developing countries, growth retardation is often associated with the quantity and /or poor quality of foods, in addition to multiple infectious hazards as previously shown [4]. It is not unlikely that aflatoxins present in human breast milk may play contributory role in growth of children in Nigeria. High levels of aflatoxin-albumin adducts have been associated with retarded growth in Beninese infants as previously shown [20,26]. Egyptian infants were reported to have stunted growth with modest maternal breast milk levels of AFM1 as previously shown [17]. Aflatoxins are genotoxic, carcinogenic substances and a threshold value below which the risk for human health is equal to zero does not exist. The joint FAO/WHO Expert Committee on Food Additives (JEFCA) does not establish a tolerable daily intake (TDI), but strongly recommended that the level of aflatoxin should be as low as possible. Therefore, the toxicological significance of the presence of aflatoxin in maternal milk should not be overlooked. Consequently a reduction of the mothers’ exposure to aflatoxins and thus a reduction of children’s exposure through breast milk are desirable. Where the source of contamination is clearly defined such as in Nigeria, post harvest treatment such as drying and storage of risk foods is an effective method, use of local spices [25-28] effective packaging and fermentation of grains [29-30].
Acknowledgment
The author wish to express her gratitude to the German Academic Exchange (DAAD) for the scholarship granted the author to carry out the work and also the host institution- The Federal University of Agriculture, Abeokuta- Nigeria for release of funds to process samples.
143
References
- Vickerman, K. (1978) Antigenic variation in trypanosomes. Nature 273: 613-617.
- Onyeyilli, P.A. and Egwu, G.O. (1995) Chemotherapy of African Trypanosomiasis: a historical perspective. ProtozoolAbst 19(5): 229- 241.
- Smith, D.H, Pepin, J, and Stich, A.H. (1998) Human African trypanosomiasis: an emerging public health crisis. Br Med Bul 54(2): 341-55.
- Nok, A.J. (2005) Effective measures for controlling trypanosomiasis. Expert OpinPharmacother 6(15): 2645-2653.
- Gutteridge, W.E. (1985). Existing chemotherapy and its limitations. Br Med Bul 41(2): 162-168.
- Atawodi, S.E., Bulus, T., Ibrahim, S., Ameh, D.A., Nok, A.J., et al. (2003) In vitro trypanocidal effect of methanolic extracts of some Nigerian Savannah plants. Afr J Biotechnol 2(9): 317-321.
- Brun, R. and Balmer, O. (2006). New developments in human African Trypanosomiasis.CurrOpin Infect Dis 19(5): 415-20.
- Hirumi, H., Doyle, J.J. and Hirumi, K. (1977) African trypanosomes: cultivation of animal-infective Trypanosome brucei in vitro. Science 196: 992-994.
- Wink, M. (1979) Trypanosomatheileri: In vitro cultivation in tsetse fly and vertebrate cell culture systems. Int J Parasitol 9: 585-589.
- Baltz, T., Baltz, D., Giround, C.H. and Crockett, J. (1985) Cultivation in a semi-defined medium of animal infective forms of Trypanosomabrucei, T. equiperdum, T. evansi, T. rhodesiense. EMBO J 4(5): 1273- 1277.
- Hirumi, H. and Hirumi, K. (1989). Continuous cultivation of Trypanosomabruceibrucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol 75(6): 985-989.
- Hirumi, H. and Hirumi, K. (1990) In vitro cultivation of Trypanosomacongolense blood stream forms in the absence of feeder cell layers. Parasitology 102: 225-236.
- Lanham, S.M. and Godfrey, D.G. (1970) Isolation of salivarian trypanosomes from man and other animals using DEAE-cellulose. ExpParasitol 28: 521-534.
- Herbert, W.J. and Lumsden, W.H. (1976). Trypanasomalbrucei: a rapid “marching method for estimating the host parasitaemia. ExpParasitol 40: 247-431
- Turgeon, M.L. (2007). Clinical laboratory science the basics and routine techniques. Mosby-Elsevier, pp 263-275.
- Jensen, J.B. and Trager, W. (1977). Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle-jar method. J Parasitol 63: 883–886.
- Scheibel, W., Ashton, S.H., and Trager, W. (1979). Plasmodium falciparum: microaerophilic requirements in human red blood cells. ExpParasitol 47: 410–418.
- Eagle, H. (1955). The specific amino acid requirement of a human carcinoma cell (Strain HeLA). J Exp Med 102: 37-48.
- Fairlamb, A.H., Operdoes, F.R., and Borst, P. (1977) New approach to screening drugs for activity against trypanosomes. Nature 265: 270- 271.
- Bossche, H.V. (1978). Chemotherapy of parasitic infections Nature 273(22): 626-10.
- Simpson, A.M., Hughes, D, and Simpson, L. (1985) Trypnosomabrucei: differentiation of in vitro grown blood stream trypomastigotes into Procyclic forms. J Parasitol 32(4): 672-677.
- Pizzolatti, M.G., Koga, A.H., Grisard, E.C., and Steindel, M. (2002) Trypanocidal activity of extract from Brazilian Atlantic Rain Forest plant species. Phytomedicine 9: 422-426.
- Shuaibu, M.N., Kanbara, T.Y., Icjinose, A., Ameh, D.A., and Nok, A.J. (2003) In vitro trypanocidal activity of dibutyltin dichloride and its fatty acid derivatives. Parasitol Res 91: 5-11.
- Huber, W. and Koella, J.C. (1993) A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 55: 257-261









