Commentary Article - (2025) Volume 0, Issue 0
Alkalinizing the Acidic Tumor Microenvironment Enhances Cancer Treatment Effectiveness
Shion Kachi1,
Reo Hamaguchi1,
Ryoko Narui11,
Hiromasa Morikawa1,
Toshihiro Okamoto2 and
Hiromi Wada1*
1Japanese Society on Inflammation and Metabolism in Cancer, Kyoto, Japan
2Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Cleveland, USA
*Correspondence:
Hiromi Wada,
Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Cleveland,
USA,
Email:
Received: 20-Jan-2025, Manuscript No. IPACLR-25-15481 ;
Editor assigned: 22-Jan-2025, Pre QC No. IPACLR-25-15481 (PQ);
Reviewed: 03-Feb-2025, QC No. IPACLR-25-15481 ;
Revised: 10-Feb-2025, Manuscript No. IPACLR-25-15481 (R);
Published:
19-Feb-2025
Introduction
Mitochondria, critical for cellular energy production, contribute to cancer progression by promoting glycolysis even in oxygen-rich conditions, a hallmark of the Warburg effect. This metabolic reprogramming enables cancer cells to maintain an alkaline intracellular pH and the acidic Tumor Microenvironment (TME), fostering tumor growth, immune evasion and therapy resistance.
Alkalinization therapy, primarily through dietary interventions, neutralizes the acidic TME to enhance cancer treatment efficacy. By consuming low Potential Renal Acid Load (PRAL) foods and alkalizing agents, systemic acidity is reduced, with urine pH serving as a practical biomarker.
Data from the Wada Clinic indicate significantly improved five-year survival rates for stage IV cancers. Notably, patients with non-small cell lung cancer exhibited a five-year survival rate nearly eight times higher than that reported by the National Cancer Center Japan. These findings suggest that incorporating pH modulation into cancer treatment represents a substantial advancement, providing a complementary approach and exploring new avenues for advanced-stage cancers.
The Tumor Microenvironment (TME) is acidic, a characteristic closely linked to the metabolism of cancer cells. In the 1920s, Otto Warburg discovered that tumor tissues metabolize approximately ten times more glucose into lactate than normal tissues, a phenomenon now known as the Warburg effect [1-5].
The Warburg effect is characterized by cancer cells generating much of their energy through glycolysis, even in sufficient oxygen. Many cancer cells shift their metabolism toward glycolysis while maintaining minimal levels of mitochondrial respiration. This metabolic trait is also suggested by the studies of Goldblatt and Cameron on oxygen deprivation and tissue culture. They discovered that intermittent oxygen deprivation promotes the formation of cancer cells and proposed that cancer development is triggered in environments rich in nutrients but deficient in oxygen [6].
Description
Generally, oxygen deprivation leads to mitochondrial disruption within cells, inducing apoptosis [7-9]. However, some cells exposed to such conditions activate a 'retrograde response' as a metabolic adaptation to hypoxia [10], allowing them to survive by relying on glycolysis [11-13].
Cancer cells exhibit unique metabolic characteristics contributing to their aggressiveness and treatment resistance. One such feature is the shift toward an alkaline intracellular pH (pHi) and the acidification of the TME [14]. In normal cells, intracellular pH is regulated by balancing proton influx and efflux through proton pumps such as the Na+/H+ Exchanger (NHE-1) [15], maintaining a slightly acidic or neutral pH. In contrast, cancer cells expel excess protons produced during glycolysis and overexpress NHE-1 transporters, maintaining an alkaline intracellular environment (pHi 7.2–7.7) while acidifying the TME [16]. In the acidic TME, cancer cells demonstrate increased motility and invasive capabilities [17]. This state promotes cellular proliferation and division, genetic abnormalities, oncogene expression, enhanced glycolytic activity, DNA synthesis, angiogenesis, metastatic potential and the acquisition of multidrug resistance in cancer cells [18-22].
The acidic TME enables immune evasion of cancer cells by suppressing the tumor immune response. The extracellular acidic environment induces the recruitment and accumulation of Myeloid-Derived Suppressor Cells (MDSCs) within the TME. Furthermore, killer T-cell activation is inhibited, regulatory T cells accumulate and the secretion of IFN-? and TNF-a is reduced. This environment also weakens the antitumor activity of natural killer and dendritic cells [23-28].
An increase in intracellular pH (pHi), or alkalization, within cancer cells has been shown to reduce the intracellular uptake of chemotherapeutic agents such as adriamycin, vinblastine and cisplatin [15,29]. Notably, research suggests that increasing the pHi from 7 to 7.4 can enhance resistance to adriamycin in human lung cancer cells by up to 2000-fold [30]. Alkalization therapy is a concept aimed at neutralizing the acidic TME, lowering cancer cells intracellular pH and inducing apoptosis. This approach has the potential to enhance cancer treatment efficacy and provides the following advantages [31,32].
• Suppression of tumor cell proliferation and metastatic ability.
• Overcoming multidrug resistance and enhancing the effectiveness of chemotherapy and immunotherapy.
• Restoration of killer T-cell and natural killer cell activity.
• Remodeling of the tumor microenvironment by suppressing the production of inflammatory cytokines.
• Metabolic reprogramming by reducing the dependence of cancer cells on glycolysis.
Alkalization therapy can overcome resistance mechanisms by targeting pH imbalances, rendering cancer cells more vulnerable to treatment. Additionally, alkalizing the TME may inhibit proton transporters like NHE-1, which play a crucial role in maintaining the alkaline intracellular environment that supports cancer cell survival and proliferation.
The alkalization therapy practiced at the Wada Clinic involves consuming alkalizing diets and bicarbonate-citric acid preparations. Specifically, this includes the intake of bicarbonates, increasing the consumption of vegetables and fruits and reducing the intake of meat and processed foods. This approach is based on the PRAL (Potential Renal Acid Load for Kidney Disease) index [33], indicating foods' acidity or alkalinity. We recommend increasing the intake of fruits and vegetables with low PRAL values, which promote alkalization, while limiting the consumption of high-PRAL foods such as animal proteins, processed foods and sugary items. Although directly measuring the acid-base balance of the TME in clinical settings is challenging, urinary pH can be used as an indirect marker. We have demonstrated favorable treatment outcomes, including improved survival prognosis and enhanced efficacy of existing chemotherapies, in cases of malignant lymphoma, gastric cancer, small cell lung cancer and breast cancer, where urinary pH alkalization was achieved [34-38].
The results of observational studies conducted over the past decade are summarized below. From January 2014 to December 2023, approximately 4,500 cases were treated at our clinic, of which 1,414 cases involved stage IV cancers across all cancer types. Among these, various analyses were conducted on 1,110 evaluable cases. Table 1 illustrates that the 5-year survival rate for stage IV cancers at the Wada Clinic was 37.7%. In contrast, the National Cancer Center Japan reported a 5-year survival rate of just 15.7% for the same time period [39], resulting in a difference of 22%. Moreover, as depicted in Figure 1, the 5-year survival rates for 700 cases treated between January 2014 and December 2018 and 410 cases treated between January 2019 and December 2023 were 35.9% and 48.8%, respectively, showing improved outcomes in the most recent 5 years. This improvement can be attributed to the accumulation of operational experience with alkalization therapy. Figure 2 compares 5-year survival rates by urinary pH. Cases with a pH > 7 had a 5-year survival rate of 50.6% (n=216), those with 6 < pH < 7 had a rate of 55% (n=127) and cases with pH = 6.0 had a significantly lower rate of 26.5% (n=67).
| |
Patients of the Wada Clinic (n) |
The 5YSR of the Wada clinic (%) |
5YSR of the National Cancer Center (%) |
| All cancers |
1110 |
37.7% |
15.7% |
| Pancreatic Cancer |
127 |
7.9% |
1.8% |
| Breast Cancer |
131 |
44.3% |
39.3% |
| NSCLC |
253 |
48.6% |
6.4% |
Note: The 5-year survival rate for stage 4 cancer was compared and examined using data from the Wada Clinic and
the National Cancer Center. The total number of cases at the Wada Clinic for all types of cancer was n=1110, with 127 cases of
pancreatic cancer, 131 cases of breast cancer and 253 cases of non-small cell lung cancer. The Wada Clinic carries out
alkalinization therapy for all types of cancer and compared to the data from the National Cancer Centre, the data showed an
improvement of around 6-42%.
Table 1: Comparison of data from the Wada Clinic and the Japan National Cancer Center.
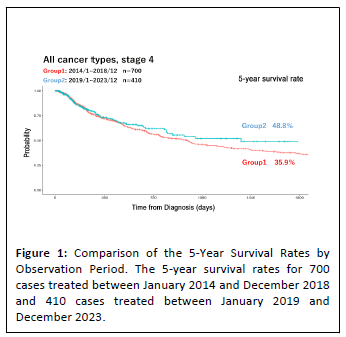
Figure 1: Comparison of the 5-Year Survival Rates by
Observation Period. The 5-year survival rates for 700
cases treated between January 2014 and December 2018
and 410 cases treated between January 2019 and
December 2023.
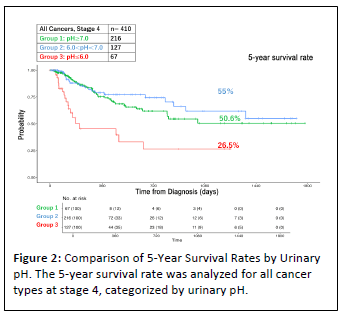
Figure 2: Comparison of 5-Year Survival Rates by Urinary
pH. The 5-year survival rate was analyzed for all cancer
types at stage 4, categorized by urinary pH.
Neutralizing the acidic TME has emerged as a promising new strategy in cancer treatment. Alkalization therapy can potentially improve treatment outcomes by suppressing tumor malignancy and restoring immune responses. While further research is necessary, this approach holds the potential to revolutionize cancer therapy, particularly when combined with chemotherapy or immunotherapy, offering the possibility of significantly improving patient prognosis.
Mitochondria are essential organelles in eukaryotic cells, responsible for energy production via Oxidative Phosphorylation (OXPHOS) [40]. Under hypoxic conditions, mitochondria cannot sustain energy production through OXPHOS and instead activate apoptosis, playing a critical role in maintaining tissue homeostasis.
Recent studies have revealed that mitochondrial dysfunction and alterations in energy metabolism are central to tumorigenesis. Specifically, signaling through "retrograde responses" from mitochondria to the nucleus induces metabolic changes, enabling cells to survive under hypoxic conditions [41-44]. This adaptation ultimately results in cancer cells that continue to produce energy through glycolysis even in the presence of oxygen, a phenomenon fundamental to the "Warburg effect".
Therefore, mitochondria play a crucial role in cancer development and progression. Their involvement in energy metabolism, apoptosis regulation and cellular adaptation to hypoxia underscores their importance in tumor biology. Understanding these mechanisms could provide valuable insights into potential therapeutic strategies targeting mitochondrial dysfunction in cancer treatment.
Detoxification within the body is considered crucial for enhancing the effectiveness of cancer treatment and reducing the aggressiveness of cancer cells. A key concept in this detoxification process is the idea of dissipative structures [45]. Dissipative structures, also referred to as 'nonequilibrium open systems,' describe the mechanism by which order emerges in living organisms, maintained by the metabolism of energy supplied from the external environment. In general, an increase in entropy, the degree of disorder, within the body can lead to disease. However, dissipative structures could decrease entropy. According to the second law of thermodynamics, entropy in an isolated system always increases. However, entropy reduction through 'reversible processes' becomes possible in nonequilibrium open systems, where energy and matter are exchanged with the environment. This mechanism allows the system to decrease overall entropy and restore order within the body under conditions of appropriate external energy supply [46]. Specifically, the human body utilizes external energy sources such as food and oxygen to maintain order. However, improper intake methods can disrupt this internal order. From this perspective, understanding the concept of dissipative structures and applying it to cancer treatment offers a novel approach to inhibiting cancer progression and provides a fresh viewpoint in therapeutic strategies.
In this context, alkalization therapy, by correcting imbalances in the body's pH, holds the potential to suppress cancer activity and synergistically enhance the efficacy of existing cancer treatments.
Conclusion
Observational studies at the Wada Clinic have demonstrated that implementing alkalization therapy through oral administration, with urinary pH as a biomarker, is associated with favorable survival outcomes in stage IV cancer patients. This approach embodies the principles of 'Science-Based Medicine' (SBM), which relies on inductive reasoning and validation based on individual cases and scientific data rather than the deductive reasoning of traditional evidence-based medicine (EBM), which draws on prior validated results.
Alkalization therapy was developed through this inductive process, focusing on the observed correlations between pH regulation, cancer progression and the clinical outcomes of patients undergoing this therapy. By challenging and complementing the methodologies of EBM, SBM integrates innovative treatments like alkalization therapy, offering new hope for patients with complex cancers. Alkalization therapy highlights the significance of metabolic and environmental factors in cancer causation, providing a holistic approach that complements conventional therapies. This integrative strategy not only aims to inhibit cancer progression but also to enhance the effectiveness of existing treatments, fostering a broader paradigm for cancer care.
Funding
The authors declare that no financial support was received for this article's research, authorship and/or publication.
Conflict of Interest
The authors declare that the research was conducted without any commercial or financial relationships that could potentially create a conflict of interest.
References
- Warburg O (1956) On the origin of cancer cells. Science 123: 309-314.
[Crossref] [Google Scholar] [Pubmed]
- Warburg O (1956) On respiratory impairment in cancer cells. Science 124: 269-270.
[Crossref] [Google Scholar] [Pubmed]
- Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. JGP 8: 519.
[Crossref] [Google Scholar] [Pubmed]
- Warburg O, Negelein E, Posener K (1924) Experiments on surviving carcinoma tissue. J Mol Med 3: 1062-1064.
[Google Scholar]
- Warburg O, Posener K, Negelein EV (1924) The metabolism of cancer cells. Biochem Z 152: 319-344.
[Google Scholar]
- Goldblatt H, Cameron G (1953) Induced malignancy in cells from rat myocardium subjected to intermittent anaerobiosis during long propagation in vitro. JEM 97: 525-552.
[Crossref] [Google Scholar] [Pubmed]
- Lagadic-Gossmann D, Huc L, Lecureur V (2004) Alterations of intracellular pH homeostasis in apoptosis: origins and roles. CDD 11: 953-961.
[Crossref] [Google Scholar] [Pubmed]
- Furlong IJ, Ascaso R, Rivas AL, Collins MK (1997) Intracellular acidification induces apoptosis by stimulating ICE-like protease activity. J Cell Sci 110: 653-661.
[Crossref] [Google Scholar] [Pubmed]
- Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC (2000) Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol 2: 318-325.
[Crossref] [Google Scholar] [Pubmed]
- Seyfried TN, Shelton LM (2010) Cancer as a metabolic disease. Nutr Metab 7: 1-22.
[Crossref] [Google Scholar] [Pubmed]
- Gatenby RA, Gillies RJ (2008) A microenvironmental model of carcinogenesis. Nat Rev Cancer 8: 56-61.
[Crossref] [Google Scholar] [Pubmed]
- Vaupel P, Schmidberger H, Mayer A (2019) The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol 95: 912-919.
[Crossref] [Google Scholar] [Pubmed]
- Vaupel P, Multhoff G (2021) Revisiting the Warburg effect: historical dogma versus current understanding. Physiol J 599: 1745-1757.
[Crossref] [Google Scholar] [Pubmed]
- Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11: 85-95.
[Crossref] [Google Scholar] [Pubmed]
- Harguindey S, Orive G, Pedraz JL, Paradiso A, Reshkin SJ (2005) The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—one single nature Biochim Biophys Acta Rev Cancer1756: 1-24.
[Crossref] [Google Scholar] [Pubmed]
- Wada H, Hamaguchi R, Narui R, Morikawa H (2022) Meaning and significance of “Alkalization Therapy for Cancer”. Front oncol 12: 920843.
[Crossref] [Google Scholar] [Pubmed]
- Gatenby RA, Gawlinski ET (1996) A reaction-diffusion model of cancer invasion. Cancer Res 56: 5745-5753.
[Google Scholar] [Pubmed]
- Harguindey S, Pedraz JL, Canero RG, de Diego JP, Cragoe EJ (1995) Hydrogen ion-dependent oncogenesis and parallel new avenues to cancer prevention and treatment using a H+-mediated unifying approach: pH-related and pH-unrelated mechanisms. Crit Rev Oncog 6: 1-33.
[Crossref] [Google Scholar] [Pubmed]
- Perona R, Serrano R (1988). Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nat 334: 438-440.
[Crossref] [Google Scholar] [Pubmed]
- Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, et al. (2000) Na+/H+ exchanger‐dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation‐associated phenotypes FASEB journal 14: 2185-2197.
[Crossref] [Google Scholar] [Pubmed]
- DiGiammarino EL, Lee AS, Cadwell C, Zhang W, Bothner B, et al. (2002) A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Mol Biol 9: 12-16.
[Crossref] [Google Scholar] [Pubmed]
- Pouysségur J (1985) The growth factor-activatable Na+/H+ exchange system: a genetic approach. Trends Biochem Sci 10: 453-455.
[Google Scholar]
- Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162-174.
[Crossref] [Google Scholar] [Pubmed]
- Petri B, Sanz MJ (2018) Neutrophil chemotaxis. Cell Tissue Res 371: 425-436.
[Crossref] [Google Scholar] [Pubmed]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, et al. (2003) Proton-sensing G-protein-coupled receptors. Nat 425: 93-98.
[Crossref] [Google Scholar] [Pubmed]
- Lattin J, Zidar DA, Schroder K, Kellie S, Hume DA, et al. (2007) G-protein-coupled receptor expression, function, and signaling in macrophages. J Leukoc Biol 82: 16-32.
[Crossref] [Google Scholar] [Pubmed]
- Wang X, Iyer A, Lyons AB, Korner H, Wei W (2019) Emerging roles for G-protein coupled receptors in development and activation of macrophages. Front Immunol 27: 2031.
[Crossref] [Google Scholar] [Pubmed]
- Stix G (2007) A malignant flame. Sci Am 297: 60-67.
[Crossref] [Google Scholar] [Pubmed]
- Gillies RJ (2001) The tumour microenvironment: causes and consequences of hypoxia and acidity. Introduction. Novartis Found Symp 240: 1-6.
[Google Scholar] [Pubmed]
- Keizer HG, Joenje H (1989) Increased cytosolic pH in multidrug-resistant human lung tumor cells: effect of verapamil. JNCI 81(9): 706-719.
[Google Scholar] [Pubmed]
- Hamaguchi R, Isowa M, Narui R, Morikawa H, Okamoto T, et al. (2024) How Does Cancer Occur? How Should It Be Treated? Treatment from the Perspective of Alkalization Therapy Based on Science-Based Medicine. Biomedicines 12: 2197.
[Crossref] [Google Scholar] [Pubmed]
- Hamaguchi R, Uemoto S, Wada H (2023) The impact of alkalizing the acidic tumor microenvironment to improve efficacy of cancer treatment. Front oncol 13: 1223025.
[Crossref] [Google Scholar] [Pubmed]
- Remer T, Manz F (1995) Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791-797.
[Crossref] [Google Scholar] [Pubmed]
- Hamaguchi R, Narui R, Morikawa H, Wada H (2021) Improved chemotherapy outcomes of patients with small-cell lung cancer treated with combined alkalization therapy and intravenous vitamin c. CDP 1: 157.
[Crossref] [Google Scholar] [Pubmed]
- Isowa M, Hamaguchi R, Narui R, Morikawa H, Okamoto T, et al. (2023) Potential of Alkalization Therapy for the Management of Metastatic Pancreatic Cancer: A Retrospective Study. Cancers 16: 61.
[Crossref] [Google Scholar] [Pubmed]
- Hamaguchi R, Isowa M, Narui R, Morikawa H, Wada H (2022) Clinical review of alkalization therapy in cancer treatment. Front oncol 14: 1003588.
[Crossref] [Google Scholar] [Pubmed]
- 37 Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, et al. (2009) Bicarbonate increases tumor pH and inhibits spontaneous metastases Cancer Res 69: 2260-2268.
[Crossref] [Google Scholar] [Pubmed]
- Ando H, Eshima K, Ishida T (2021) Neutralization of acidic Tumor Microenvironment (TME) with daily oral dosing of sodium potassium citrate (K/Na Citrate) increases therapeutic effect of anti-cancer agent in pancreatic cancer xenograft mice model Biol Pharm Bull 44: 266-270.
[Crossref] [Google Scholar] [Pubmed]
- Japan, C (2024) Cancer Information Service, National Cancer Center, Japan.
- Stoldt S, Wenzel D, Kehrein K, Riedel D, Ott M, et al. (2018) Spatial orchestration of mitochondrial translation and OXPHOS complex assembly. Nat Cell Biol 20: 528-534.
[Crossref] [Google Scholar] [Pubmed]
- Jazwinski SM, Kriete A (2012) The yeast retrograde response as a model of intracellular signaling of mitochondrial dysfunction. Front physiol 3: 139.
[Crossref] [Google Scholar] [Pubmed]
- Butow RA, Avadhani NG (2004) Mitochondrial signaling: the retrograde response. Mol Cell 14: 1-5.
[Crossref] [Google Scholar] [Pubmed]
- Jazwinski SM (2014) The retrograde response: A conserved compensatory reaction to damage from within and from without. PMBTS 127: 133-154.
[Crossref] [Google Scholar] [Pubmed]
- Jazwinski SM (2013) The retrograde response: When mitochondrial quality control is not enough. Biochim Biophys Acta Mol Cell Res 1833: 400-409.
[Crossref] [Google Scholar] [Pubmed]
- Prigogine I (1978) Time, structure, and fluctuations. Science 201: 777-785.
[Crossref] [Google Scholar] [Pubmed]
- Ben-Amotz D, Honig JM (2006) The rectified second law of thermodynamics. J Phys Chem 110: 19966-19972.
[Crossref] [Google Scholar] [Pubmed]
Citation: Kachi S, Hamaguchi R, Narui R, Morikawa H, Okamoto T, et al. (2025) Alkalinizing the Acidic Tumor Microenvironment Enhances Cancer
Treatment Effectiveness. Ann Clin Lab Vol.13 No.S5:002
Copyright: © 2024 Kachi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which
permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.








