Serah Funke Ige1*, Mayowa Jeremiah Adeniyi2 and Grace Oladuuni Iyalla1
1Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria
2Department of Physiology, University of Benin, Benin, Nigeria
*Corresponding Author:
Serah Funke Ige
Department of Physiology, Faculty of Basic Medical Sciences
College of Health Sciences, Ladoke Akintola University of Technology
Ogbomoso, Oyo State, Nigeria
Tel: +2348060743616
E-mail: sfige@lautech.edu.ng
Received date: August 01, 2017; Accepted date: August 05, 2017; Published date: August 15, 2017
Citation: Ige SF, Adeniyi MJ, Iyalla GO. Allium cepa Mitigates Aluminum Chloride-Induced Hepatotoxicity in Male Wistar Rats. J Biomedical Sci. 2017; 6(4):27. doi: 10.4172/2254-609X.100071
Copyright: © 2017 Ige SF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Hepatotoxicity; Allium cepa; Catalase; Aspartate transaminase; Alanine transaminase bilirubin
Introduction
Within the ecosystem, interaction between living organisms and environmental borne factors such as dusts and chemicals is inevitable [1]. Human exposure to chemicals, most especially aluminum occurs through natural processes such as weathering of rocks and erosion [2]. Dietary and occupational exposures to the mineral are also sources of aluminum in humans and animals [2,3]. During normal breathing, at least 1.4 μg of aluminum in particulate was reported to be inhaled per day [3]. With a significant aluminum load, the excretory capacity of the kidney is exceeded leading to deposition of the minerals in various tissues including the liver and consequently resulting in hepatotoxicity [2,4,5].
In animal studies, experimental hepatotoxicity was associated with alterations in liver function test such as total plasma protein, plasma liver enzymes (ALT, AST and alkaline phosphatase) and plasma bilirubin [6-8]. A study by Ige et al. (2011) [6] on cadmium-induced hepatic damage showed a decrease in total serum protein and significant increases in serum ALT and AST activities in male rats. Arsenic and mercury loading were also associated with increases in plasmatic activities of AST, ALT and alkaline phosphatase in rats [7,9]. Interestingly, a high oxidant/antioxidant ratio was a common feature characterizing these reports, indicating the plausible role of oxidative stress in the liver damage.
Amelioration of damage and prevention of diseases have always been linked with nutritional status and dietary factors [10]. Many food stuffs especially flavonoid-containing types have been shown to play important role in the prevention of tissue damage and management of diseases [11,12]. Allium cepa, an example of flavonoid-containing vegetable [13] has been shown to exhibit high therapeutic efficacy against atherosclerotic conditions and organ toxicity in cadmiumintoxicated rats [14]. It also prevented obesity related malignancy [15] and averted hyperglycemia in diabetic rats [16]. We have demonstrated in our previous study that Allium cepa ameliorated aluminum chloride induced reproductive dysfunctions in male rats [17]. The present study was designed to investigate the effect of Allium cepa administration on oxidative stress-mediated hepatoxicity in male wistar rats following aluminum chloride exposure.
Materials and Methods
Animals care and management
Twenty adult male wistar rats weighing 175 g were used for the research work. They were divided into four groups consisting of five animals each. These rats were kept in four different cages with a wire mesh covering. They were fed pelletized grower’s mash ad libitum, provided water through drinking trough and kept under 12 hour light and 12 hour darkness at room temperature.
Ethical certification
The study was conducted in line with the guidelines of United State National Institute of Health (NIH) Guidelines for the use of laboratory rats.
Experimental procedure
The rats were weighed and randomly grouped into;
Group A: received distilled water for four weeks.
Group B: received a single daily administration of 100 mg/kg body weight of AlCl3 (P.O.) for four weeks
Group C: received a single daily administration of 1 ml/0.1 kg body weight of Allium cepa (P.O) for four weeks.
Group D: received single daily administrations of 100mg/kg body weight of AlCl3 (P.O.) and 1 ml/0.1 kg body weight of Allium cepa (P.O.) for four weeks.
Preparation of Allium cepa extract
Allium cepa was prepared following procedures from previous studies [6,14]. Briefly, fresh common onion (Allium cepa) bulbs were rinsed thoroughly in distilled water, air dried, and 200 g was then blended. The resulting paste was allowed to stand for 24 hrs. Juice was then filtrated and squeezed out of it using a tight sieve. The filtrate/juice was prepared on weekly basis following the same procedure and kept at 4°C. This is to prevent it from losing its potency.
Sample collection
At the end of the experiment, blood samples were collected via cardiac puncture. Livers of rats were harvested, weighed and homogenized in phosphate buffer for determination of oxidative stress.
Determination of total serum protein, total serum bilirubin, and serum liver enzymes
Total serum protein and bilirubin were assayed using a standard enzymatic calorimetric method with standard laboratory kits (LABKIT, Barcelona-Spain) as reported by Ige et al. (2011) [6]. Serum liver enzymes (ALT and AST) were also determined using standard kit (Biosystems S.A Barcelona-Spain).
Determination of liver superoxide dismutase (sod), catalase activities and malondialdehyde (mda) levels
At the end of the experiment, the livers were excised, cleaned and weighed. A portion of each liver was washed in normal saline (0.9% NaCl), homogenized in phosphate buffer (1 g tissue/ 4ml). The homogenate were then centrifuged and aliquots of the supernatant were obtained for biochemical analysis. Liver SOD, catalase activities and MDA levels were determined as reported by Fridovich (1986) [18], Sinha (1972) [19] and Varshney and Kale (1990) [20] respectively.
Statistical analysis
Results are presented as mean ± SEM. Significant difference was set at P<0.05. Data were analysed by using a one-way analysis of variance (ANOVA) followed by unpaired Student’s ttest.
Result
Effect of Allium cepa on the liver weight of Aluminum treated rats
Figure 1 shows the liver weight across the groups. AlCl3 treated group has non-significant increase liver weight while Allium cepa, Al+Allium cepa and control liver weight were comparable.
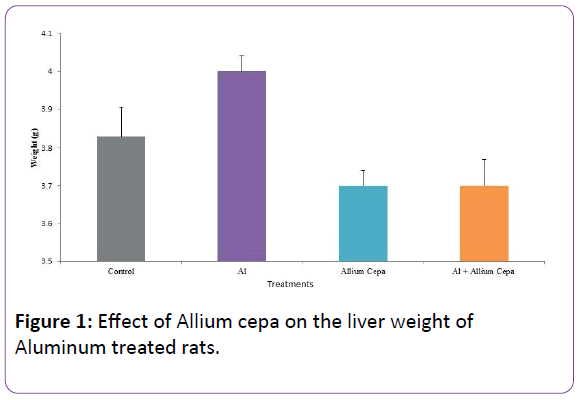
Figure 1: Effect of Allium cepa on the liver weight of Aluminum treated rats.
Effect of Allium cepa on some liver function parameters
There was significant decrease serum total protein level and increase serum total bilirubin, ALT and AST in the AlCl3 treated group when compared with control. The Allium cepa alone treated group showed a significant increase in serum total protein level and decrease serum total bilirubin, ALT and AST when compared with control group. There was no significant difference in serum total protein level, serum total bilirubin, ALT and AST of Al+ Allium cepa group when compared with control (Table 1).
| Parameters |
Control |
Al |
Allium cepa |
Al+Allium cepa |
| Total Serum Protein (g/dl) |
35.75 ± 2.55 |
26.08 ± 2.92* |
60.9 ± 3.66* |
30.2 ± 1.00 |
| Total Serum Bilirubin (mg/dl) |
0.82 ± 0.02 |
1.02 ± 0.02* |
0.68 ± 0.02* |
0.84 ± 0.01 |
| ALT(IU/L) |
18.3 ± 0.30 |
28.12 ± 3.75* |
16.4 ± 6.40* |
17.5 ±0.50 |
| AST(IU/L) |
54 ± 0.32 |
60.6 ± 0.400 |
50.6 ± 0.40 |
53 ± 0.32 |
*p=0.05 vs. control
Table 1: Effect of Allium cepa on some liver function Parameters.
Effect of Allium cepa on some Antioxidant Activities and Hepatic Lipid peroxidation
Aluminum administration caused a significant decrease in SOD, catalase activities and increase in MDA level (marker of lipid peroxidation). While Allium cepa treatment caused significant increase in SOD, catalase and decrease MDA. There was no significant difference in SOD, catalase and MDA of Al +Allium cepa animals when compared with the control (Figure 2 and Table 2).
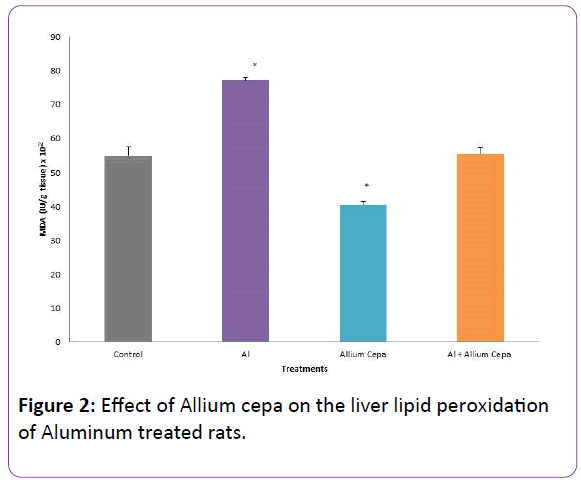
Figure 2: Effect of Allium cepa on the liver lipid peroxidation of Aluminum treated rats.
| Antioxidants |
Control |
Al |
Allium cepa |
Al+Allium cepa |
| SOD (IU/g tissue) |
1.29 ± 0.02 |
0.75 ± 0.03* |
1.66 ± 0.04* |
1.33 ± 0.01 |
| CAT (IU/g tissue) × 103 |
15 ± 0.58 |
6 ± 0.57* |
19 ± 5.7* |
13 ± 1.5 |
*p,0.05 vs. control
Table 2: Effect of Allium cepa on some antioxidant activities.
Discussion and Conclusion
Liver, one of the largest organs in the body [21,22] plays an indisputable role in catabolic and anabolic processes, storage, synthesis and detoxification [21-23]. Alteration in liver function test is characterized by increase in serum ALT, AST, ALP, total bilirubin and liver weight and decrease in total protein [24,25]. The present study demonstrated that administration of AlCl3 led to hepatotoxicity. Many studies have documented that administration of cadmium, arsenic acid and AlCl3 impaired liver function [6,7,8,26]. The change in serum ALT, ALP, bilirubin and total protein levels in this study clearly indicated a significant damage of hepatocytic cell membranes [4] resulting in leakage of these chemical substances into the systemic circulation and attenuation of synthetic capacity of the organ.
There was an insignificant rise in liver weight of AlCl3 group. This is inconsistent with previous study [27] where significant increase in liver weight was observed. Dissimilarity observed in this study might be due to the short duration of Al treatment. There was also comparable liver weight in control, Allium cepa alone and AlCl3+Allium cepa rats. This confirms the non-toxic effect of Allium cepa as earlier reported [28].
In this study, the least activity of antioxidant enzymes (SOD and catalase) and the highest level of MDA were observed in AlCl3 group. MDA, a reactive metabolite, produced during peroxidation of polyunsaturated fatty acid tends to bombards tissue borne molecules [29,30], resulting in a high MDA/ antioxidant enzyme ratio, oxidative stress and tissue deterioration. As far as this study is concerned, AlCl3 induced hepatotoxicity was mediated by oxidative stress.
Researchers have found that Allium cepa essential oil, Allium cepa flesh and Allium cepa juice exerted a positive influence on health [11,12,31]. The present study corroborated this report. We noticed a decrease in ALT, AST, total protein and an elevated total protein in ACE group. On the other hand, the hepatic tissue displayed a low level of MDA and high activities of SOD and catalase. This indicated that the positive influence of Allium cepa on liver function biomarkers was mediated by a low MDA/ antioxidant enzyme ratio.
Studies have reported the beneficial effect of Allium cepa on arsenic acid induced- and cadmium-induced hepatic damage in rats [5-7,26]. Exposure of human beings to both Allium cepa and aluminum chloride is insurmountable [2]. The present study showed that there was an insignificant change in ALT, AST, total bilirubin and total serum protein in Allium cepa+AlCl3 group. This implied that administration of Allium cepa may exert a mitigating effect on the hepatotoxic effect of AlCl3. Also, we observed that there was no change in hepatic MDA and hepatic antioxidant enzymes. This also indicated that the mitigating influence of Allium cepa involves restoration of hepatic MDA and hepatic antioxidant. However; subsequent study will be needed to clarify the mode of action of Allium cepa on aluminum chloride-induced hepatotoxicity. In conclusion, the result of the study showed that Allium cepa mitigated aluminum chloride-induced hepatotoxicity through regulation of hepatic oxidant/antioxidant system.
Acknowledgments
The authors are highly thankful to the Dr. Jelili A. Badmus, Department of Biochemistry for his assistance during the biochemical analysis study.
20186
References
- Odum EP (1971) “Fundamentals of Ecology” (Third ed.). New York: Saunders.
- Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, et al. (2007) Human health Risk Assessment for Aluminum, Aluminum Oxide and Aluminum Hydroxide”. J Toxicol Environ Health B Crit Rev 1: 1-269.
- Exley C (2013) Human exposure to aluminum. Environ Sci Process Impacts 15: 1807-1816.
- Klaassen CD, Watkin JB (1984) Mechanism of bile formation, hepatic uptake and biliary excretion. Pharmacol Rev 36: 1-67.
- Buraimoh AA, Ojo SA, Hambolu JO, Adebisi SS (2012) Effects of Aluminium Chloride Exposure on the Histology of the Liver of Adult Wistar Rats. IOSR Journal of Pharmacy 2: 525-533.
- Ige SF, Akhigbe RE, Edeogho O, Ajao FO, Owolabi OQ, et al. (2011) Hepatoprotective Activities of Allium cepa in Cadmium-Treated Rats. Int J Pharm Pharm Sci 3: 60-63.
- Messarah M, Klibet F, Boumendjel A, Abdennour C, Bouzerna N, et al. (2012) Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp. Toxicol Pathol 64: 167-174.
- Chong-sheng B, Han-song Z (2012) Effects of Subchronic Aluminum Exposure on Liver Function in Rats. J Northeast Agric. Univ 19: 652-662.
- El-Shenawy SM, Hassan NS (2008) Comparative Evaluation of the Protective Effect of Selenium and garlic against liver and kidney damage induced by mercury chloride in rats. Pharmacol Rep 60: 199-208.
- Conlin PR (1999) the dietary approaches to stop hypertension (Dash) clinical trial: implications for lifestyle modifications in the treatment of hypertensive patients. Cardiology Review 7: 284-288.
- Park J, Kim J, Kim MK (2007) Onion flesh and onion peel enhance antioxidant status in aged rats. J Nutr SciVitaminol 53: 21-29.
- El-Demerdash FM, Yousef MI, El-Naga NI (2005) Biochemicalstudy on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol 43: 57-63.
- Rodriguez GB (2008) Rodriguez Rodriguez EM and Diaz RC. Flavonoids in onion cultivars (Allium cepa L.). J Food Sci 73: 599-605.
- Ige SF, Akhigbe RE (2013) Common onion (Allium cepa) extract reverses cadmium-induced organ toxicity and dyslipidaemia via redox alteration in rats. Pathophysiology 20: 269-274.
- Wang Y, Tian WX, Ma XF (2012) Inhibitory. Effects of Onion (Allium cepa L.) Extract on Proliferation of Cancer Cells and Adipocytes via Inhibiting Fatty Acid Synthase. Asian Pacific J Cancer Prev 13: 5573-5579
- Ojo RJ, Opara PE, Babatunde PF (2015) Comparative Effect of Daily Administration of Allium sativum and Allium cepa Extracts on Alloxan Induced Diabetic Rats. IOSR journals 1: 26-31.
- Ige SF, Akhigbe RE (2012) The role of Allium cepa on aluminum-induced reproductive dysfunction in experimental male rat models 5: 200-205
- Fridovich I (1986) Superoxide dismutases. Adv Enzymel Relat Area Mol Biol 58: 61-97.
- Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47: 389-394.
- Varshney R, kale RK (1990) Effects of Calmodulin Antagonists on Radiation-Induced Lipid Peroxidation Microsomes. Int J Radiat Biol 58: 733-743.
- Elias H, Bengelsdorf H (1952) the Structure of the Liver of Vertebrates. Acta Anat (Basel) 14: 297-337.
- Abdel-Misih SR, Bloomston M (2010) Liver Anatomy. Surg Clin North Am 90: 643-653.
- Tortora GJ, Derrickson BH (2008) Principles of Anatomy and Physiology. John Wiley & Sons.
- David E, Johnston MD (1999) Special considerations in interpreting liver function tests. Am Fam Physician 59: 2223-2230.
- McClatchey KD (2002) Clinical Laboratory Medicine. Lippincott Williams & Wilkins.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160: 1-40.
- Ighodaro OM, Omole JO, Ebuehi OA, Salawu FN (2012) Aluminium-induced liver and testicular damage: effects of Piliostigma thonningii methanolic leaf extract. Nig Q J Hosp Med 22: 158-163.
- Enitan SS, Ajeigbe KO, Josiah SJ, Ehiaghe FA (2012) Haematological and Hepatotoxic Potential of Onion (Allium cepa) and Garlic (Allium sativum) Extracts in Rats. European Journal of Medicinal Plants 2: 290-307.
- Davey MW, Stals E, Panis B, Keulemans J, Swennen RL (2005) High-throughput determination of malondialdehyde in plant tissues. Analytical Biochemistry 347: 201-207.
- Mo J, Xia Y, Wade TJ, Schmitt M, Le XC (2006) Chronic arsenic exposure and oxidative stress: OGG1 expression and arsenic exposure nailselenium, and skin hyperkeratosis in Inner Mongolia. Environ Health Perspect 114: 835-841.
- El-Soud NA, Khalil M (2010) Antioxidative Effects of Allium cepa Essential Oil in Streptozotocin Induced Diabetic Rats. Maced J Med Sci 3: 344-351.







