Keywords
|
| HMGB1; Pyruvate kinase; Glutamine; Autophagy; Skeletal muscle |
Introduction
|
| The amino acid balance that normally maintained in healthy people becomes altered in patients of various diseases due to metabolic changes. Recently, metabolism has been shown to be completely altered in cancer cells [1]. The Warburg effect is the most well-known alteration of metabolism involving aerobic glycolysis in cancer cells. This alteration of metabolism affects the plasma free amino acid (PFAA) profile. Therefore, this altered PFAA profile could be a good marker of various cancers. We focused high motility group box (HMGB)-1. HMGB1 is a recent focus of cancer research. HMGB1 play pivotal roles in cancer development, progression, and metastasis by activation of cancer cells, enhance of tumor angiogenesis, and inhibition of host anticancer immunity. HMGB1 is binding to receptor for advanced glycation end products (RAGE) and toll-like receptor (TLR)4 in cancer cells and monocyte-lineage immune cells. HMGB1 induces apoptosis in monocyte-lineage immune cells; HMGB1 inhibits tumor-infiltrating macrophages and dendritic cells, lymph node sinus macrophages, liver Kupffer cells, and lymph node dendritic cells to attenuate anti-cancer immune responses and antimetastatic organ defense. In this article, the role of HMGB1 in mechanism of the alteration of PFAAs was explained. |
|
Plasma free amino acids profile in cancer
|
| The PFAA profile is affected by various pathological conditions. Japanese patients with metabolic syndromes show altered PFAA profiles; however, the reduction of body mass index, blood pressure, and levels of hemoglobin A1c and triglycerides were shown to normalize the PFAA profile [2]. |
| Changes in protein metabolism, as reflected by the PFAA profile, may represent a new diagnostic tool for cancer [3]. It is conceivable that, based on the understanding of the differences in PFAA profiles in cancer, an increasing amount of rational and more specific antineoplastic strategies may arise. |
| In a study by Miyagi et al., plasma samples were collected from approximately 200 patients diagnosed with lung, gastric, colorectal, breast, or prostate cancer [4], and the patients were compared to gender and age-matched controls. Univariate analysis revealed significant differences in the PFAA profiles between the controls and the patients with any of the five types of cancer listed above, including those with asymptomatic early-stage disease. Furthermore, multivariate analysis clearly discriminated the cancer patients from the controls (AUC of ROC >0.75 for each cancer) regardless of the cancer stage. These findings suggest that PFAA profiling has great potential for aiding the screening and diagnosis of early stage cancer as well as understanding disease pathogenesis [4]. |
| In reviewing the PFAA profiles of lung cancer patients, a significant reduction of the gluconeogenic amino acids threonine, serine, and glycine and a significant increase of tryptophan and glutamate were found [5]. In breast cancer patients, a significant increase of ornithine, glutamate, and tryptophan was found. This comparison of PFAA profiles between lung and breast cancer suggests that different tumors have different influences on patients’ PFAA patterns [5]. |
| As reported by Lee et al. [6], about the one third of early and the late stage colorectal cancer patients had a body weight loss more than 10% in half a year and were defined as having malnutrition. For individual amino acids in early stage colorectal cancer patients, most of the PFAAs are decreased (significantly in Tyr, Ala, Met, Phe, and Thr). In late stage colorectal cancer patients, the plasma levels of most essential amino acids and non-essential amino acids decreased more profoundly. For group amino acids, the plasma levels of essential amino acids, nonessential amino acids, gluconeogenic amino acids, and branched chain amino acids were also lower in the cancer patients than in control volunteers. This difference was also noticeably significant in patients with late stage colorectal cancer. Furthermore, the PFAA profiles in colorectal cancer patients are considerably different from those in patients with non-gastrointestinal cancer and weight loss. The plasma level of essential amino acids and branched-chain amino acids was not kept within the normal range in colorectal cancer patients [6]. |
|
Plasma free amino acids index - A sensitive marker for cancer detection
|
| Okamoto et al., examined the PFAA-based index for the detection of colorectal cancer [1]. The index is inferred by multivariate logistic regression analysis and classifier composed with six amino acids (Glu, Gly, ABA, His, Trp, and Arg), which AUC of ROC is 0.812 in the study data and 0.768 in the test data. Further analysis demonstrated that the index could discriminate colorectal cancer patients equally in any stage, and particularly in patients with stage 0, I, and II cancer, of which there are few known tumor markers. The distribution of the index is independent of known tumor markers; therefore, higher detection efficiency would be expected by combinatorial use of the index and tumor markers. |
| The levels of several PFAAs are significantly different in endometrial cancer, which provides the specific PFAA index [7]. The AUC of ROC is 0.94, which greatly distinguishes endometrial cancer patients from control subjects. The AUC for the index is significantly larger than that for CA125. For the same specificity of 98.3%, the index shows a significantly higher sensitivity (60%, 95% CI, 43.3-75.1) than that of CA125 (22.5%, 95% CI, 10.1- 38.5). In stage I cases of endometrial cancer, the index shows a significantly higher positivity than that of CA125. |
|
Glutamine - A major abnormal element of PFAA
|
| Glutamine is the most abundant, free alpha-amino acid in plasma and skeletal muscle [8]. This nutrient plays an important role in regulating gene expression, protein turnover, anti-oxidative function, nutrient metabolism, immunity, and acid-base balance. |
| Glutamine, traditionally considered a nonessential amino acid, now is considered "conditionally essential" after critical illness [9]. States of illness can lead to a significant decrease in the plasma levels of glutamine, and when this decrease is severe, it has been correlated with increased mortality. Laboratory data have demonstrated the numerous benefits of glutamine in experimental models of critical illness, cancer, and cardiac injury. The mechanism of these protective effects includes attenuated proinflammatory cytokine expression, improved gut barrier function, enhanced ability to mount a stress response, improved immune cell function, and decreased mortality. |
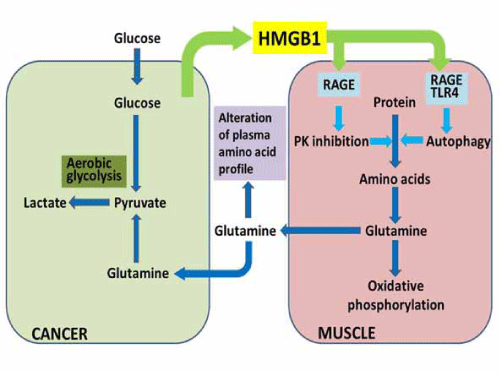
| Plasma glutamine is increased in colorectal cancer, which is confirmed by PFAA profiling [1,4]. These glutamine plasma concentrations are associated with disease progression and plasma HMGB1 concentration [10]. Glutamine in the plasma is absorbed by cancer cells and integrated into the TCA cycle after glutaminolysis, and then generates pyruvate that is used in lactate fermentation [10]. Plasma glutamine is provided from the skeletal muscle by HMGB1-induced autophagy [10]. This cancer-muscle relationship through glutamine is advocated by DeBeradinis [11]. We confirmed this relationship and identified HMGB1 as the key factor [10]. Amino acid lysis linked with autophagy is induced under HMGB1 existing conditions. Glutamine is normally used as a source of TCA cycle intermediates including acetyl-CoA in the muscle tissue. However, tumor cells used glutamine as a source of pyruvate for lactate fermentation. HMGB1, which is secreted from cancer cells, is a key tool for cancer cells to usurp energy from the muscle tissue. |
|
HMGB1 - A disturbing agent of intramuscular metabolism
|
| HMGB1 is a multifunctional protein that plays a role as a growth factor and enhances proliferation, invasion, and metastasis by binding to the receptor for advanced glycation endproducts (RAGE) in various cancers [12,13]. HMGB1 is released by necrotic cells or secreted from monocyte-lineage cells and induces inflammation based on innate immunity [14,15]. An excess concentration of HMGB1 induces apoptosis in monocyte lineage cells to reduce anti-cancer immunity and enhance cancer metastasis [13]. HMGB1 has been shown to play a pivotal role in the carcinogenesis and progression of colorectal cancer [16- 18]. In a rat model of colon carcinogenesis using azoxymethane, HMGB1 levels continuously increased in the colonic mucosa [18]. Several reports have shown that HMGB1 activates autophagy. HMGB1 binds to the toll-like receptor 4 (TLR-4) to activate innate immunity and immunological autophagy by activating Beclin-1 via dissociation from B-cell leukemia/lymphoma 2 (Bcl-2) [19,20]. Cytosolic HMGB1 also directly interacts with Beclin-1 to dissociate it from Bcl-2 [21]. |
| HMGB1 treatment decreases pyruvate kinase (PK) M1 production in mouse skeletal muscle which does not produce PKM2 [10]. PK is responsible for the linker reaction between glycolysis and the TCA cycle, followed by oxidative phosphorylation. PKM1 is expressed in mature tissues, whereas PKM2 is expressed in embryonic tissues and cancer cells. Expression switching from PKM1 to PKM2 is due to c-Myc activation [22]. Decreased levels of PKM1 result in lower PK activity. PK inactivation decreases the pyruvate supply and the supply of downstream acetyl-CoA; however, mitochondrial energy production is still maintained [10]. To compensate for the decreased production of acetyl-CoA from pyruvate, glutaminolysis might be induced, which supplies 2-oxoglutarate to the TCA cycle. The results of carbon chase experiments indicate that glutamine is metabolized to produce acetyl-CoA primarily, and the TCA cycle is able to continue to produce energy [10]. |
| HMGB1 induces the dephosphorylation of mTOR, which upregulates autophagy-associated Beclin-1 and LC3 through RAGE-associated p38 phosphorylation [10]. In pancreatic cancer cells, RAGE is shown to induce sustained autophagy and to suppress apoptosis [23]. Moreover, HMGB1 activates TLR-4 to release Beclin-1 from the complex with Bcl-2. TLR-4- induced autophagy is associated with defense against microbes, inflammation, and tumorigenesis [19,20]. Thus, HMGB1 induces proteolysis by autophagy, which may supply free amino acids. An autophagy-induced glutamine supply may potentially be important to maintain the mitochondrial energy production in the muscle [10]. Bluemlein et al., has described that PK maintains amino acid homeostasis, and low PK increases cellular glutamine [24] (Figure 1). |
|
Plasma free amino acids profile and HMGB1
|
| HMGB1 induces autophagy, which is used to maintain aerobic energy production. The PFAA profile is altered due to autophagy, amino acid lysis, and amino acid utilization in the TCA cycle [10]. These findings suggest that the PFAA profile might be a sensitive probe of altered host metabolism due to cancer development. Indeed, HMGB1 levels are increased from the early stages of cancer in a mouse model of colon carcinogenesis [10,18]. The PFAA profile is confirmed to be altered in mice during colon |
| carcinogenesis. Importantly, mice in this model show an altered PFAA profile even prior to forming neoplasms [10]. A cluster analysis showed that PFAA profiles of early carcinogenesis model mice, untreated mice, anti-HMGB1 antibody-treated carcinogenesis-model mice, and PBS-injected mice belonged to the same cluster. In contrast, late carcinogenesis-model mice, control serum-treated carcinogenesis-model mice, and HMGB1- injected mice belonged to the other cluster [10]. These results suggest that HMGB1 is the most prominent factor discriminating the alteration of PFAA profile in these experimental systems. |
|
Amino acid targeting - A new strategy of cancer treatment
|
| Glutamine targeting is performed using an inactive glutamine analog, 6-diazo-S-oxo-L-norleucine (DON) or glutaminase knockdown. These treatments decrease glutamine uptake into cancer cells and inhibit cancer growth in a mouse subcutaneous tumor model [10]. Glutamine targeting is reported to be effective in suppressing cancer metastasis [25]. While cancer cells are consuming glucose and glutamine as energy sources, the targeting of glutamine alone is effective enough to suppress cancer growth. Differing from glucose, glutamine is essential to DNA synthesis [26]. Its targeting is relevant in inhibiting energy production and cell duplication. Otherwise, glutamine targeting possesses a meaning of correction to an abnormal pattern of the PFAA profile. For example, autophagy-induced malnutrition causes compromised immunity and cancer progression, and tolerance to anti-cancer treatment might be diminished [27]. To avoid excess catabolic changes in cancer-burdened hosts with altered energy production, an adequate amino acid supply could be important. The necessity of supplying branched-chain amino acids has been proposed [27]. Current evidence does not necessarily predict a special need or role for glutamine supplementation in critical illness. However, glutamine supplementation may have some benefits during cancer chemotherapy [28]. The dose and route of administration clearly influence the benefit observed from glutamine administration [9]. |
Conclusion
|
| The PFAA profile suggests altered energy production in cancer-burdened hosts. Cancer-secreted HMGB1 is responsible for the alteration of PFAA in cancer. Understanding the novel relationship between cancer and the skeletal muscle might provide a perspective for improve the quality of life in cancer hosts. |
Figures at a glance
|
 |
| Figure 1 |
|
| |
| |






