Mushfiquddin Khan1*, Tajinder Singh Dhammu1, Tejbir Singh Dhaindsa1, Hamza Khan2, Avtar K Singh1,3 and Inderjit Singh1
1Department of Pediatrics, Medical University of South Carolina, Charleston, SC, USA
2Faculty of Medicine, University of South Carolina, Columbia, SC, USA
3Department of Pathology and Laboratory Medicine, Medical University of South Carolina, Charleston, SC, USA
*Corresponding Author:
Mushfiquddin Khan
Ph.D., Associate Professor, Medical University of South Carolina, Department of Pediatrics, Charleston, SC 29425, USA
Tel: (843) 792-7991
Fax: (843) 792-3653
E-mail: khanm@musc.edu
Received Date: December 10, 2015; Accepted Date: December 28, 2015; Published Date: December 31, 2015
Citation: Khan M, Dhammu TS, Dhindsa TS, et al. An NO/GSNO-based Neuroregeneration Strategy for Stroke Therapy. J Neurol Neurosci. 2016, 6:4. DOI: 10.21767/2171-6625.100058
Keywords
Stroke; GSNO; Neuroregeneration; S-Nitrosylation; Functional recovery; HIF-1α; Angiogenesis
Introduction
Stroke is the leading cause of serious, long-term disability in the USA at great burden to families and medical care agencies. Furthermore, it is associated with significant morbidity/mortality and a high cost of approximately $50-65 billion annually in the USA [1]. In developed countries, the incidence of stroke is declining, largely due to successful efforts at lowering blood pressure and reducing smoking. However, the overall rate of stroke remains high due to increases in aging populations. Nearly three-quarters of all strokes occur in people over the age of 65. The risk of having a stroke more than doubles each decade after the age of 55 years. Therefore, age is recognized as the most significant non-modifiable risk factor for stroke. In the aged population, the mechanisms of stroke disease are distinct from stroke mechanisms in the younger population. Older stroke survivors have not only compromised neuroprotection mechanisms but also slower rates of neuroregeneration. Improvement by a therapeutic agent of these rates of neuroregeneration and neurobehavioral functions will determine the efficacy and the clinical relevance of a therapy in stroke. Neuroregeneration mechanisms are significantly dependent on the reduction of nitro-oxidative stress, and these mechanisms are reported to be stimulated by GSNO following stroke injury. Therefore, the aim of this article is to highlight that GSNO stimulates neuroregeneration mechanisms via the stabilization of the HIF-1α/VEGF pathway in the chronic phase of stroke disease.
GSNO is a natural component of the human body produced by the reaction of NO with glutathione (GSH) in the presence of oxygen [2]. It is present in the brain and other organs [3]. GSNO is directly involved in cell signaling via S-nitrosylation of target proteins, including NF-κB, STAT3, COX-2, caspase-3, calpains, inducible nitric oxide synthase (iNOS), and endothelial NOS [4-7]. Exogenous administration of GSNO [8] also protects against cardiac ischemic injury [9,10], supporting the therapeutic potential of GSNO. Pharmacological inhibition of GSNO reductase (GSNOR) has also been shown to improve endothelial functions [11], indicating a protective role of GSNO in neurovascular dysfunction-related diseases. Studies have reported that GSNO inhibits platelet activation in humans [12] and protects both BBB integrity and epithelial permeability [13,14].
In a microenvironment of stroke injury, NO released by conventional NO-donors or NO gas itself is immediately inactivated by superoxide, forming peroxynitrite. This disadvantage of inactivation is not associated with the S-nitrosylating agent GSNO. In addition, S-nitrosylation of cysteine residue (a reversible modification) prevents it from further oxidation to sulfinic and sulfonic acids (an irreversible modification), thereby preventing inactivation of both NO and proteins. Neurorepair from GSNO may be mediated by two different mechanisms: 1) S-nitrosylation and 2) maintaining redox by mechanistically reducing the production of oxidants, including peroxynitrite. This multi-mechanistic functional and therapeutic potential is not embedded in conventional NO donors, making GSNO a unique candidate to be investigated for the stimulation of functional recovery following stroke. Furthermore, GSNO therapy can be initiated even late with or without the administration of the FDA-approved tissue plasminogen activator (tPA). GSNO is inexpensive, readily available, and GSNO’s exogenous administration in humans or animals has not resulted in toxicity or side effects.
GSNO activates Akt via S-nitrosylation-dependent inhibition of PTEN [15]. PTEN is a lipid phosphatase, which regulates cellular phosphatidylinositol phosphate levels and PI3 kinase signaling. S-nitrosylation of PTEN results in inhibition of its activity, leading to the activation of Akt [16]. Inhibition of GSNO-mediated Akt activation reverses GSNO-mediated neuroprotection and functional recovery [17]. Activation of Akt is reported to stabilize hypoxia-inducible factor-1 alpha (HIF-1α), which, in turn, induces VEGF and angiogenesis [18]. HIF-1α is also stabilized by its direct S-nitrosylation, and S-nitrosylation-mediated stabilization of HIF- 1α has been reported to increase angiogenesis in a myocardial injury model [10], indicating an overall regenerative role of S-nitrosylated HIF-1α. There is a close relationship between angiogenesis/neurogenesis and VEGF. Increased neurogenesis is accompanied by increased angiogenesis, whereas angiogenesis up regulates neurotrophic factors. Angiogenesis itself is regulated by VEGF, mainly via HIF-1-based transcription.
Hippocampal neuronal cell loss following IR is linked to cognitive alterations, leading to impairments in learning and memory. The adult mammalian brain generates new neurons (neural progenitor cells; NPC) in the hippocampus, dentate gyrus (DG), and subventricular zone (SVZ). These progenitors possess the ability to self-renew, differentiate into mature neurons, and enhance plasticity. Restorative neurogenesis occurs after stroke, but a majority of the new cells die as a result of the oxidative environment, resulting in limited and insufficient functional recovery. Therefore, the focus of functional recovery research in stroke is dual pronged: the induction of neurogenesis and protection against oxidative/ inflammatory loss [19]. GSNO treatment of stroke confers not only this neuroprotection against neuroinflammatory insults but also induces angiogenesis via stimulating VEGF through the stabilization of the HIF-1α/VEGF pathway. VEGF has been shown to modulate coupling of angiogenesis and neurogenesis; hence, it is essential for neuroregeneration [20]. GSNO-mediated increased cell proliferation and vessel density correlating with functional recovery in a 2-week IR study [21] further support the neurodegenerative role of GSNO. Based on neuroprotective efficacy and neuroregenerative ability, GSNO has strong potential as a drug candidate for evaluation in human stroke. However, certain precautions are associated with the use of GSNO as therapy due to its structural configuration. GSNO is comparatively less stable compound and its metabolism is affected by several factors including light, temperature, metals and enzymes [22]. The activity of GSNO is also associated with hypotension [23]; therefore, physiologic parameters must be monitored if higher doses of GSNO are used. In our studies, freshly prepared (in dark) GSNO (1-3 μM/kg body weight) is slowly (10-15 min) infused in experimental animals via jugular vein or tail vein. This protocol of GSNO preparation and administration had no any significant change in physiologic parameters [24]. Currently, we are investigating the efficacy of GSNO for functional recovery in permanent ischemic stroke animal models (Figure 1).
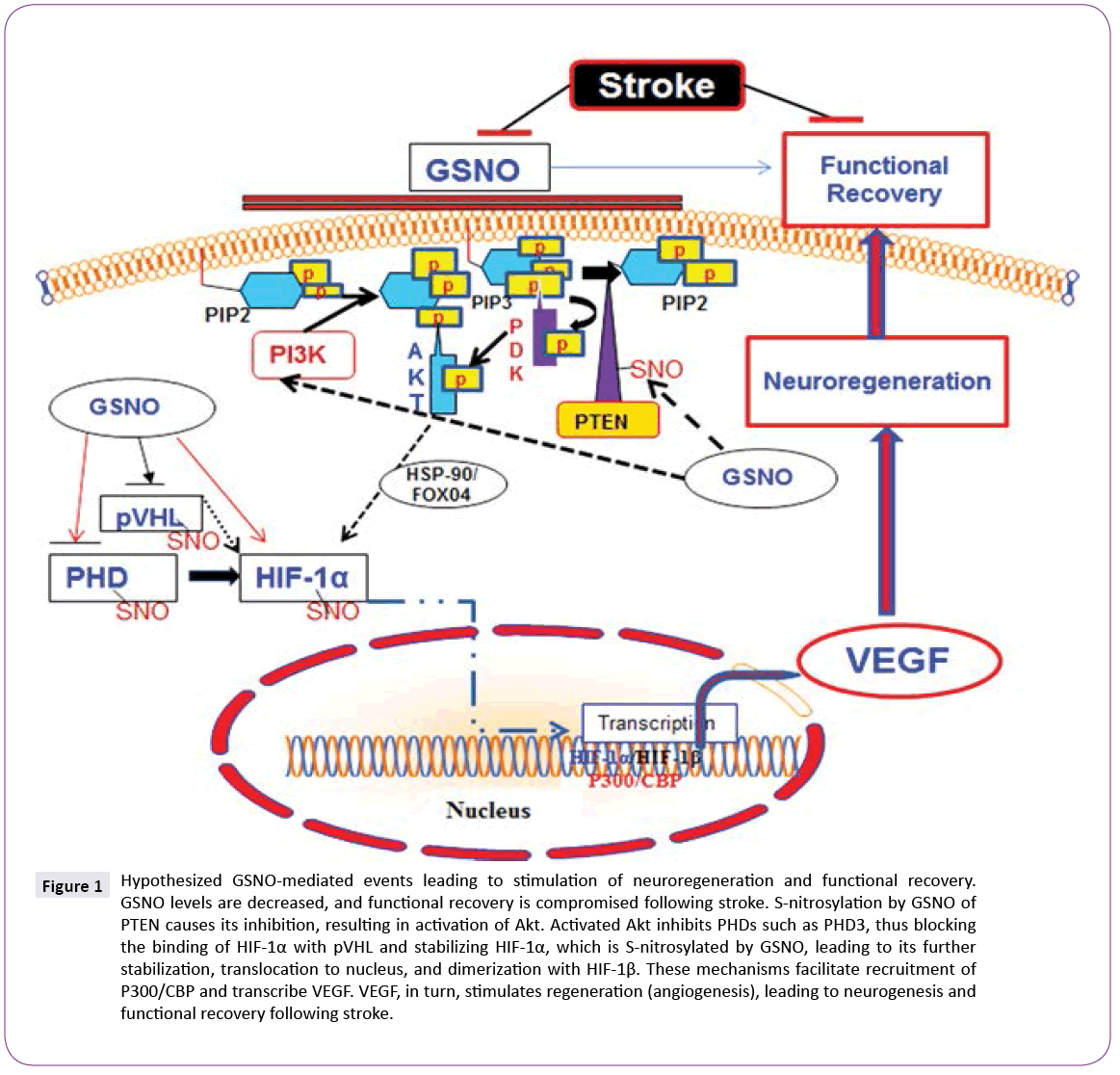
Figure 1: Hypothesized GSNO-mediated events leading to stimulation of neuroregeneration and functional recovery. GSNO levels are decreased, and functional recovery is compromised following stroke. S-nitrosylation by GSNO of PTEN causes its inhibition, resulting in activation of Akt. Activated Akt inhibits PHDs such as PHD3, thus blocking the binding of HIF-1α with pVHL and stabilizing HIF-1α, which is S-nitrosylated by GSNO, leading to its further stabilization, translocation to nucleus, and dimerization with HIF-1β. These mechanisms facilitate recruitment of P300/CBP and transcribe VEGF. VEGF, in turn, stimulates regeneration (angiogenesis), leading to neurogenesis and functional recovery following stroke.
Conclusion
HIF-1α stabilization-based intervention in ischemia using inhibitors of its hydroxylating enzyme prolyl hydroxylases (PHDs) has provided neuroprotection and stimulated neurorecovery in a number of preclinical studies [25], indicating that HIF-1α stabilization by GSNO/S-nitrosylation is a logical target for stroke treatment. GSNO is an endogenous neuroregeneration-inducing agent, and its exogenous administration protects against both acute and chronic phase injuries following experimental stroke. Furthermore, no toxicity or side effects were reported following its administration in humans [12,26,27] for other indications or in animals [28]. Therefore, investigating the potential of S-nitrosylation mechanism using GSNO as a therapeutic agent in stroke is a promising approach with clinical implications. We propose that an administration of low dose GSNO is an ideal strategy to stimulate neuroregeneration mechanisms for functional recovery in stroke patients.
Acknowledgements
This work was supported by grants from NIH (NS-72511). We acknowledge Dr. Tom Smith from the MUSC Writing Center for his valuable editing of the manuscript.
Conflict of Interests
The authors declare that they have no competing interests.
8108
References
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, et al. (2008) Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25-146.
- Singh SP, Wishnok JS, Keshive M,Deen WM, Tannenbaum SR (1996) The chemistry of the S-nitrosoglutathione/glutathione system. ProcNatl Acad Sci U S A 93: 14428-14433.
- Kluge I, Gutteck-Amsler U, Zollinger M, Do KQ (1997) S-Nitrosoglutathione in Rat Cerebellum: Identification and Quantification by Liquid Chromatography-Mass Spectrometry. J Neurochem 69: 2599-2607.
- Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH (2001) Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3: 193-197.
- Khan M, Dhammu TS, Sakakima H, Shunmugavel A, Gilg AG, et al. (2012) The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke. J Neurochem 123 Suppl 2: 86-97.
- Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, et al. (2005) S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab 25: 177-192.
- Kim J, Won JS, Singh AK, Sharma AK, Singh I (2013) STAT3 Regulation by S-Nitrosylation: Implication for Inflammatory Disease. Antioxid Redox Signal.
- Rassaf T, Poll LW, Brouzos P, Lauer T, Totzeck M, et al. (2006) Positive effects of nitric oxide on left ventricular function in humans. Eur Heart J 27: 1699-1705.
- Konorev EA, Tarpey MM, Joseph J, Baker JE, Kalyanaraman B (1995) S-nitrosoglutathione improves functional recovery in the isolated rat heart after cardioplegic ischemic arrest-evidence for a cardioprotective effect of nitric oxide. J Pharmacol Exp Ther 274: 200-206.
- Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, et al. (2009) Endogenous S-nitrosothiols protect against myocardial injury. ProcNatlAcadSci U S A 106: 6297-6302.
- Chen Q, Sievers RE, Varga M, Kharait S, Haddad DJ, et al. (2013) Pharmacological inhibition of S-nitrosoglutathionereductase improves endothelial vasodilatory function in rats in vivo. J ApplPhysiol 114: 752-760.
- Radomski MW, Rees DD, Dutra A, Moncada S (1992) S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol 107: 745-749.
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, et al. (2007) Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132: 1344-1358.
- Khan M, Im YB, Shunmugavel A, Gilg AG, Dhindsa RK, et al. (2009) Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J Neuroinflammation 6: 32.
- Leslie NR (2006) The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal 8: 1765-1774.
- Numajiri N, Takasawa K, Nishiya T, Tanaka H, Ohno K, et al. (2011) On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN). ProcNatlAcadSci U S A 108: 10349-10354.
- Sakakima H, Khan M, Dhammu TS, Shunmugavel A, Yoshida Y, et al. (2012) Stimulation of functional recovery via the mechanisms of neurorepair by S-nitrosoglutathione and motor exercise in a rat model of transient cerebral ischemia and reperfusion. RestorNeurolNeurosci 30: 383-396.
- Cheng XW, Kuzuya M, Kim W, Song H, Hu L, et al. (2010) Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation 122: 707-716.
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, et al. (2003) Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol 53: 743-751.
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, et al. (2008) Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab 28: 764-771.
- Khan M, Dhammu TS, Matsuda F, Baarine M, Dhindsa TS, et al. (2015) Promoting endothelial function by S-nitrosoglutathione through the HIF-1alpha/VEGF pathway stimulates neurorepair and functional recovery following experimental stroke in rats. Drug Des Devel Ther 9: 2233-2247.
- Broniowska KA, Diers AR, Hogg N (2013) S-nitrosoglutathione. BiochimBiophysActa 1830: 3173-3181.
- Zanini GM, Martins YC, Cabrales P, Frangos JA, Carvalho LJ (2012) S-nitrosoglutathione Prevents Experimental Cerebral Malaria. J Neuroimmune Pharmacol 7: 477-487.
- Khan M, Jatana M, Elango C, Paintlia AS, Singh AK, et al. (2006) Cerebrovascular protection by various nitric oxide donors in rats after experimental stroke. Nitric Oxide 15: 114-124.
- Harten SK, Ashcroft M, Maxwell PH (2010) Prolyl hydroxylase domain inhibitors: a route to HIF activation and neuroprotection. Antioxid Redox Signal 12: 459-480.
- deBelder AJ, MacAllister R, Radomski MW, Moncada S, Vallance PJ (1994) Effects of S-nitroso-glutathione in the human forearm circulation: evidence for selective inhibition of platelet activation. Cardiovasc Res 28: 691-694.
- Hornyak I, Pankotai E, Kiss L, Lacza Z (2011) Current developments in the therapeutic potential of S-nitrosoglutathione, an endogenous NO-donor molecule. Curr Pharm Biotechnol 12: 1368-1374.
- Colagiovanni DB, Borkhataria D, Looker D, Schuler D, Bachmann C, et al. (2011) Preclinical 28-day inhalation toxicity assessment of s-nitrosoglutathione in beagle dogs and wistar rats. Int J Toxicol 30: 466-477.






