Keywords
Solid organ injury; Pediatric blunt; Trauma centers; Blunt abdominal Trauma
Introduction
The invention ofa vaccine is a complex and challenging process, which differs form the development ofo rdinary medicines. The normal time period for development of a vaccine is 12-15 years [1-3]. While the ordinary medicines are oriented towards the treatment of a disorder whose symptoms have arisen, vaccines are prepended for use in persons not yet exhibiting diseasem anifestation, in order to prevent the occurrence of diseases.
The invention and production of a corona virus vaccine is a critical issue, but it is likely to take many months to resolve. Although many companies have announced that the corona virus vaccine will be ready soon, this will be sophisticated to do in reality [4].
Current Global Situation
The procedure of developing safe and effective vaccines involves a number of stages, from the laboratory to the people. The first tsep opf re-clinical testing involves scientists administering the vaccine to animals such as mice or monkeys, and observing if it produces an immune response. There are currently more than 125 vaccines undergoing pre-clinical testing for COVID-19 (Figures 1-3). The vaccine will then enter three phases of clinical trials [5-9] (Table 1).
| Phase |
Goal |
| Pre-clinical trial |
Studies of the vaccine candidate in the laboratory or in non-human (animals). |
| Phase-1 clinical trial |
Studies of fewer than 100 subjects (people) to assess safety of vaccine candidate. |
| Phase-2 clinical trial |
Studies of several hundred people to preliminarily test safety and immune response. |
| Phase-3 clinical trial |
Studies of thousands of people to formerly test safety and efficacy |
| Phase-4 clinical trial/Post marketing surveillance |
Ongoing studies of the vaccine after it has been approved. |
Table 1: Phase of clinical trial.
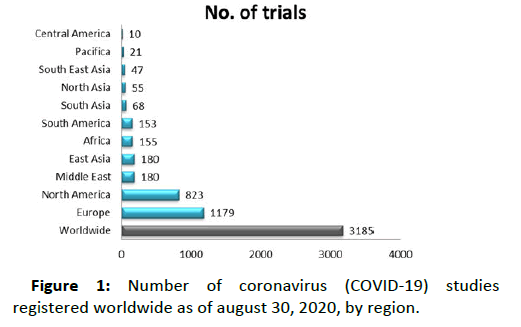
Figure 1: Number of coronavirus (COVID-19) studies registered worldwide as of august 30, 2020, by region.
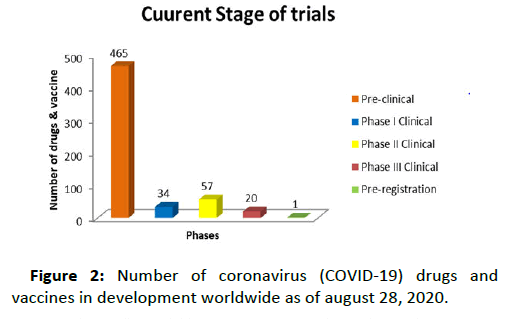
Figure 2: Number of coronavirus (COVID-19) drugs and vaccines in development worldwide as of august 28, 2020.
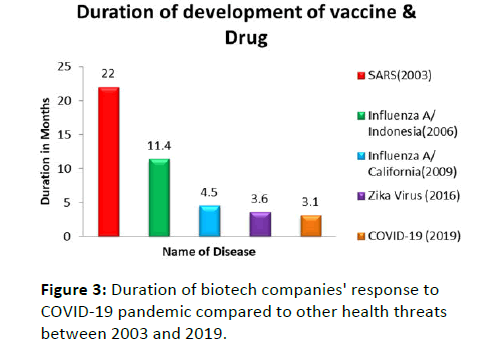
Figure 3: Duration of biotech companies' response to COVID-19 pandemic compared to other health threats between 2003 and 2019.
Type of Vaccines Against SARS-COV-2
RNA vaccines: The mRNA vaccine encodes a stable prefused form of spike protein (S). Protein complex S is required for membrane fusion and host cell infection and has been the target of vaccines against MERS and SARS [10].
DNA vaccines: DNA vaccines showing the modern direction of development in the production of vaccines. Vaccines obtained by recombinant DNA technology are produced by genetic moderation .A clinical trial for a new DNA vaccine against COVID-19 has already begun. This is called ChAdOx1 nCoV-19 and it was initially developed to prevent Middle East Respiratory Syndrome [11,12].
Protein subunit: Subunit vaccines contain only certain antigenic determinants of pathogenic microorganisms, and are obtained either starting from traditional cultivation processes, or by recombinant DNA technology [13].
Viral vectors: Along with traditional virus vaccines, viral vectors are widely used, in which genome of one virus is used to deliver the antigen of another virus, thus allowing development of a platform technology of virus production [14].
VLPs (Virus-Like Particles): Vaccine development based on the recombinant proteins and Virus-Like Particles (VLPs) is a more innovative apporach. Antiviral vaccines are usually developed on the basis of surface proteins that form VLPs. Production of VLPs in the cells with further reconstruction into the stable and immunogenic forms is a multi-stage process [15].
Inactivated virus: These vaccines containing whole microorganisms, but inactivated by chemical or physical methods. Vaccine have higher stability; however, effectiveness is lower and requires reminders of immune system [16].
Live attenuated virus: Live attenuated vaccines were the first vaccines utilized. These types of vaccines are obtained through cultivation of microorganisms under suboptimal conditions or through successive passage in cultures, techniques that determine attenuation of virulence while maintaining the capacity to induce the immune response [17] (Table 1).
COVID-19 Vaccine Development Updates by Countries
Rapid production of a vaccine to prevent coronavirus disease 2019 (COVID-19) is a global imperative, and defining the stakes and potential hurdles is critical because regulatory and medical decisions are based on benefit and risk. Analyzing LOS, we found that all centers reduced their median LOS by 1 to 1.5 days [18]. However, all centers fell short of abbreviated bedrest protocols stipulated by guidelines. In 2014, examining the first decade of NOM protocols, Dodgion et al. noted that LOS was lower compared to APSA guidelines and higher than those suggested by St. Peter’s bedrest protocol, suggesting hybrid adaptations among trauma centers [20].
mRNA-1273 (United States)
mRNA-1273 was developed by Moderna based on prior studies of related coronaviruses such as those that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). A Phase 1 trial (of 105 healthy participants provided the basis for Moderna’s investigational new drug application which was successfully reviewed by the FDA and set the stage for Phase 2 testing. A Phase 2 trial of 600 healthy participants evaluating 25 μg, 100 μg, and 250 μg dose levels of the vaccine was completed, and mRNA-1273 has advanced to a Phase 3 trial A Phase 3 trial of 30,000 participants at high risk for SARS-CoV-2 infection who will receive a 100 μg dose of mRNA-1273 or placebo and then followed for up to 2 years. On 12 May, the FDA granted Fast Track designation to mRNA-1273. A Phase 3 trial of the vaccine is underway, which is being funded by Operation Warp Speed [18,19].
Inactivated vaccine by sinopharm (China)
Inactivated vaccine for COVID-19 sponsored by Wuhan Institute of Biological Products, China National Pharmaceutical Group (Sinopharm) presently on Phase 3 registered under Henan Provincial Center for Disease Control and Prevention . Researchers from China have initiated a randomized, doubleblind, placebo parallel-controlled Phase 1/2 clinical trial of healthy individuals starting at 6 years old. An outcome of the vaccine has shown a "strong neutralizing antibody response" in Phase 1/2 trials, according to a release from China National Biotec Group. The outcome from Phase 1 and Phase 2 trial show the vaccine candidate has demonstrated immunogenicity. Presently a Phase 3 trial is underway in Peru, Morocco, and in the United Arab Emirates [20].
AZD1222 (AstraZeneca) (United States)
The Oxford Vaccine Group at the University of Oxford are developing a new vaccine candidate for COVID-19, a chimpanzee adenovirus vaccine vector called AZD1222 (previously ChAdOx1). In India, the candidate is being jointly developed by the Serum Institute of India and AstraZeneca, and goes by the name Covishield. Preclinical data showed a significantly reduction in viral load and “humoral and cellular immune response.” A Phase 1/2 single-blinded, multi-center study of 1,090 healthy adult volunteers aged 18-55 years with four treatment arms. A Phase 3 trial enrolling up to 30,000 participants is underway. Preliminary results showed “acceptable safety profile” with most patients demonstrating an antibody response after one dose and all patients showing a response after two doses. Phase 3 trials are being conducted in the United States and in study sites in India [21].
Ad5-nCoV (China)
China’s CanSino Biologics has developed a recombinant novel coronavirus vaccine that incorporates the adenovirus type 5 vector (Ad5). Preliminary safety data from a Phase 1 clinical trial of 108 participants between 18 and 60 years old who will receive low, medium, and high doses of Ad5-nCoV has allowed the company to plan to initiate a Phase 2 trial. Outcomes from Phase 1 of the trial show a humoral and immunogenic response to the vaccine, In Phase-2 of the trial, neutralizing antibodies and specific interferon γ enzyme-linked immunospot assay responses were observed at all dose levels for most participants. Updates on 25 June, China’s Central Military Commission announced the military had been approved to use Ad5-nCoV for a period of 1 year, according to reporting in Reuters. A Phase 3 trial in Saudi Arabia is currently underway [19].
BNT162 (North America)
BNT162 was initially four vaccine candidates originally developed by BioNTech, two candidates consisting of nucleoside modified mRNA-based (modRNA), one of uridine containing mRNA-based (uRNA), and the fourth candidate of self-amplifying mRNA-based (saRNA). The companies have selected the modRNA candidate BNT162b2 to move forward in a Phase 2/3 trial. A Phase 1/2 trial in the US and Germany of 200 healthy participants between aged 18-55 years, with a vaccine dose range of 1 μg to 100 μg is currently recruiting as is a Phase 2/3 trial of about 32,000 healthy participants. On 20 August, outcome showed similar immunogenicity between BNT162b1 and BNT162b2 but fewer adverse effects with BNT162b2. BNT162b2 was selected to advance to a Phase 2/3 safety study "based on the totality of available data from our preclinical and clinical studies, including select immune response and tolerability parameters." A candidate could be ready for regulatory approval as early as December.
INO-4800 (Korea)
INO-4800 is a DNA vaccine candidate matched to the novel coronavirus SARS-CoV-2. which causes the COVID-19 disease in humans. The INO-4800 vaccine contains the plasmid pGX9501, which encodes for the full length of the Spike glycoprotein of SARS-CoV-2. Preclinical data showed that mice and guinea pigs who received INO-4800 demonstrated neutralizing antibodies as well as humoral and T cell responses. In guinea pigs, researchers observed protein binding antibody titers and blocking of angiotensin-converting enzyme 2 (ACE2)/SARS-CoV-2 S proteins. Outcome of INO-4800 reduced viral load in both the lower lungs and nasal passages in macaques that received two doses of INO-4800 (1 mg) four weeks apart and then were challenged with live virus 13 weeks after the second dose (study week 17). After 4 months, durable antibody and T cell responses were observed in the animals as well as memory T and B cell responses [22].
Picovacc vaccine (China, Jiangsu)
Inactivated novel coronavirus vaccine (PiCoVacc) is the Beijing-based Sinovac Biotech’s vaccine was able to induce specific neutralizing antibodies in all three animal models. CoronaVac (formerly PiCoVacc) is a formalin-inactivated and alum-adjuvanted candidate vaccine. Outcome from animal studies showed “partial or complete protection in macaques” exposed to SARS-CoV-2, the vaccine is currently undergoing human clinical trials. In April, the company commenced phase-1 trial on 144 healthy adults aged 18-59 years. The outcome of phase 1/2 trials indicate the vaccine has good safety and immunogenicity, with 92.4% of participants receiving the 3 μg dose on a 0-14 day schedule and 97.4% of individuals receiving the dose on a 0-28 day schedule achieving seroconversion. Currently Sinovac said a Phase 3 trial in collaboration with Instituto Butantan in Brazil is underway [23].
NVX-CoV2373 (Australia, Queensland)
NVX-CoV2373 is a prefusion protein coronavirus vaccine candidate made using Novavax’s proprietary nanoparticle technology, Matrix-M, which is an adjuvant to enhance immune responses and stimulate high levels of neutralizing antibodies. Novavax identified NVX CoV2373 as its lead SARS-CoV-2 candidate following pre-clinical testing that demonstrated high immunogenicity and high levels of neutralizing antibodies. A Phase 1 clinical trial of NVX CoV2373 initiated in Australia during May 2020. On 4 August, Novavax announced positive Phase 1 results for NVX-CoV2373 indicating participants who had received the vaccine developed an antibody response in participants. Novavax plans to manufacture 1 billion doses of NVX-CoV2373 by 2021 as part of their recent acquision of Praha Vaccines [24,25].
Sputanik v (Russia)
Sputnik V, a human adenoviral vector vaccine that fights against coronavirus disease. It is developed by the Gamaleya National Research Institute of Epidemiology and Microbiology. Russian researchers informed that presently the vaccine in phase 3 clinical trial. Sputnik V – formerly known as Gam-COVIDVac and developed by the Gamaleya Research Institute in Moscow and approved by the Ministry of Health of the Russian Federation on 11 August. Phase I-II clinical trials of Sputnik V showed no serious adverse events (SAE, Grade 3) for any of the criteria. In 100% of participants in the clinical trials, Sputnik V generated a stable humoral and cellular immune response. A number of countries, such as UAE, Saudi Arabia, Philippines and possibly India or Brazil will join the clinical trials of Sputnik V locally. Mass production of the vaccine is expected to start in September 2020 [26].
Covaxin (India)
India’s first potential COVID-19 vaccine has been given approval for Phase I and Phase II clinical trials. Named COVAXIN™, the vaccine was developed by Bharat Biotech and has been granted approval from the Drugs Controller General of India (DCGI). Human clinical trials are scheduled to start across India in July 2020. Bharat Biotech created COVAXIN in collaboration witht he Indian Council of Medical Research (ICMR) and National Institute ofV irology (NIV). The DCGI granted permission to initiate Phase I and II human clinical trials after the company submitted results generated from pre-clinical studies, demonstrating “promising” and “extensive” safety and immune responses. Dr Krishna Ella, Chairman and Managing Director of Bharat Biotech, said: “We are proud to announce COVAXIN, India’s first indigenous vaccine against COVID-19. The collaboration withI CMR and NIV was instrumental in the development of this vaccine [27].
Conclusion
Scientists began working on COVID-19 vaccines during SARS outbreak, but their attempts did not materialize because of innumerous hassles. Since coronavirus pandemic, the spread of the outbreak appears much broader than was the case for SARS. There is also the possibility of the disease becoming endemic and seasonal in its appearance, according to some investigators. This unfolds why many researchers and pharmaceutical agencies are undertaking vigorous attempts to invent and develop a potent vaccine against SARS-CoV-2 all over the world, also speeding up all the usual stages needed to develop and tryout a vaccine in the human.
36695
References
- Han S (2015) Clinical vaccine development. Clin Exp Vaccine Res 4: 46-53.
- Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, et al. (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19: 305-306.
- Number of coronavirus (COVID-19) studies registered worldwide as of August 30, 2020, by region.
- Number of coronavirus (COVID-19) drugs and vaccines in development worldwide as of August 28, 2020, by phase.
- Duration of biotech companies' response to COVID-19 pandemic compared to other health threats between 2003 and 2019.
- Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F (2021) The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines. 5: 112020.
- Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, et al. (2011) Clinical applications of DNA vaccines: Current progress. Clin Infect Dis 53: 296–302.
- Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, et al. (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19: 305-306.
- Zhang N, Tang J, Lu L, Jiang S, Du L (2015) Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res 202: 151–159.
- Choi Y and Chang J (2013) Viral vectors for vaccine applications. Clin Exp Vaccine Res 2: 97-105.
- Sarkar, Bishajit, Islam, Syed Zohora, Umme Ullah, Md. Asad (2019) Virus like particles: A recent advancement in vaccine development. Korean J Microbiol 55: 327-343.
- Sridhar S, Brokstad KA, Cox RJ (2015) Influenza vaccination strategies: Comparing inactivated and live attenuated influenza vaccines. Vaccines (Basel) 3: 373–389.
- Lauring AS, Jones JO, Andino R (2010) Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol 28: 573–579.
- Clinical Trails (2020) Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) US.
- Xia S, Duan K, Zhang Y, Chen W, You W, et al. (2020) Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes:Interim analysis of 2 randomized clinical trials.JAMA 324: 951-960.
- Wu S, Zhong G, Zhang J,Shuai L, Zhang Z, et al.(2020) A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge.Nat Commun4081.
- Pharmaceuticals I, Innovations C (2020) Safety, tolerability and immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers.
- Safety and Immunogenicity Study of Inactivated Vaccine for Prophylaxis of SARS CoV-2 Infection (COVID-19) 2020.
- Keech C, Gary A, Cho IMS, Robertson A, Reed P, et al. (2020) Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med 383:2320-2332.
- Novavax (2020) Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant.
- Clinical trial of efficacy, safety, and immunogenicity of gam-COVID-Vac vaccine against COVID-19 (RESIST) (2020).
- Victoria Rees (2020) First COVID-19 vaccine clinical trial approved in India. Eur Pharm Rev.









