Background: Multiple sclerosis (MS), which commonly affects the young population, is a demyelinating disease of immunological origin. MS-related pain occurs either directly as a result of inflammation of neural tissue, or as a result of muscle spasms and spasticity exerting pressure on the musculoskeletal system. Cannabis has proven ability to manage pain associated with MS. Central pain is a chronic syndrome that occurs commonly in multiple sclerosis patients, with a frequency of patients affected ranging from 36% to 82%. Central pain is one of the most common symptoms that affect the quality of life of MS patients only second to fatigue.
Objective: To systematically evaluate the literature comparing the analgesic effects of pharmacological treatments for Trigeminal Neuralgia in patients with MS.
Methods: The research was conducted using a search strategy to identify randomized clinical trials on pharmacological treatments of central pain in MS. The clinical trials were found in the following databases: Pub med (1965-2014), Cochrane (2000-2014), Lilacs (2000-2014), Proquest (2000-2014), Medline (1965- 2014) with latest search date December 2013. Selection criteria: Randomized controlled trials with subjective pain assessment using the Jadad assessment score including a score greater than three for selection criteria.
Results: Seven studies met inclusion criteria and were analyzed. Six of them compared cannabinoids against placebo. One compared the anticonvulsant lamotrigine versus placebo. Randomized studies of cannabinoids against placebo found a decrease of three points in the Jadad pain scale with treatments using Dronabinol (cannabinoids). Patients reported mild adverse effects including: dizziness, drowsiness, dry eyes and impaired balance. These symptoms did not compromise the quality of life of patients. The overall conclusive treatment effect of central pain in MSwith Lamotrigine was not significant (p=0.67).
Conclusions: Most of the prospective clinical trials evaluating the pharmacologic treatment of central pain in MS, involved trials using cannabinoids. Cannabis reduces pain in MS sufferers by directly working to reduce immune response and resultant inflammation. There appears to be a statistically significant analgesic effect in patients with MS-related central pain. Clinical trials with larger samples are needed in order to provide stronger evidence regarding the safety and effectiveness of therapeutic doses of cannabinoids. There is a lack of well-designed studies aimed at the other drug therapies for MS-related central pain.
Keywords
Multiple sclerosis; Central pain; Adverse effects; Cannabinoids; Anticonvulsants
Introduction
Multiple Sclerosis (MS) is an autoimmune inflammatory disease that affects the central nervous system (CNS) including the presence of axonal degeneration and focal lesions in the white matter [1,2]. MS affects approximately 350,000 individuals in the United States and more than one million individuals worldwide [3-6]. Although the true onset of the disease is before the clinical symptoms, MS typically is found between the ages of 18 and 45 years. The risk of developing MS is approximately 1 per 1000 approximately (0.1%) in the general population. This risk increases from 20 to 40 per 1000 (2%-4%) when a first-degree relative is affected by MS [3]. The most common symptoms of the disease include, mainly trigeminal neuralgia, tonic spasms and spasticity, and central pain.
Central pain is defined as "pain initiated or caused by a primary lesion or dysfunction of the central nervous system (CNS)" by the International Association for the Study of Pain (IASP). Recently, another definition has been introduced, suggesting that central pain is "pain that arises as a direct result of an injury or disease affecting the central level somatic sensory system" [7-9]. Following this new definition, the prerequisites for a definitive diagnosis of central pain is that it should have a neuro-anatomical distribution and it should arise from an injury or disease that affects the relevant somatic sensory system. Interestingly, central pain affects 53% to 86% of patients diagnosed with MS as such pain is one of the major factors that compromising quality of life in this patients [10-12].
Prior reviews have shown the effectiveness of drugs including anticonvulsants, antidepressants and cannabinoids in the treatment of central pain. In particular, there are randomized controlled trials comparing the effectiveness of cannabinoids against placebo. However, clinical trials showing a comparison between different drug groups that could help distinguish which is the most effective drug for central pain control in MS patients with the least side effects are scares [13-16]. Accordingly, the aim of the present review was to examine the literature to shed light into the effectiveness of pharmacological agents in the treatment of central pain. We evaluated the current prospective, randomized clinical trials evaluating the efficacy of drugs in central pain in MS using a systematic review and describe by statistical analysis the most efficient medication with the least side effects.
Central pain and multiple sclerosis
Pain is a common and distressing symptom associated with MS. The incidence of MS related pain varies from 53% to 86% and is often sub-optimally managed [4]. The occurrence of pain, grade of severity, description and location were reported in different articles [17,18]. Females constitute the majority of the population of MS [5,19] numbers in terms of gender have been changing over the last 20 years and the experience of pain is widespread [20]. The management of MS pain has been analyzed through the representative scale of pain which is not determined by sex and is rather generalized for all types of patients [21].
Current studies evaluating the efficacy of the anticonvulsants, antidepressants and cannabinoids have shown efficacy in decreasing the severity of chronic pain. Drugs that have been studied in pain associated with MS include Lamotrigine, Gabapentin, Amitriptyline and Dronabinol.
Methods
This is a systematic review of the literature to evaluate prospective, randomized, controlled clinical trials. The selection criteria were studies published in peer-review journals in the term of January 2000 to December 2014. Randomized studies have evaluated the pharmacologic effect of cannabinoids, anticonvulsants, opioids, antidepressives in the treatment of central pain in MS. The exclusion criteria were studies with power less of 80% and studies evaluating neuropathic pain or somatic pain (Table 1). Medline, EMBASE, PsycINFO, LILACS, Web of Science, and Scopus were used in the research. The search yielded a total of 1,681 published articles. We examined the listed conditions and excluded non-neurologic studies. Surveys, case reports/series, and non–placebo-controlled trials were also excluded. Of the 1,681 abstracts reviewed, we reviewed the full text of 68 articles and found that seven met inclusion criteria. Some articles were used to answer more than pain control also reporting more common side effects. Multiple outcomes were adjusted by the researches panel with a Bonferroni correction, unless otherwise specified.
| Inclusion Criteria |
Exclusion Criteria |
| Studies published between January 2000 and December 2014. |
Studies evaluating neuropathis or somatic pain. |
| Items belonging to indexed publications. |
Preliminary studies in a pilot or sample sizes with power <80%. |
| Randomized placebo-controlled clinical trials. |
Items that have no primary information of the medication (eg dose). |
| Articles on the treatment of central pain in patients with multiple sclerosis. |
Items not evaluate pain based on a scale of measurement. |
| Articles written in English. |
|
| Items which assess pain intensity before and after treatment. |
|
| Articles evaluated with the Jadad scale with results greater than or equal to three. |
|
Table 1 Inclusion and Exclusion Criteria.
Results
Sixty-eight articles were evaluated, forty were excluded on the basis of clinical design and not were controlled. Of the selected articles, twenty-one additional studies were excluded owing to the treatment of neuropathy as primary outcome-chronic pain and not central pain in MS. Seven articles were found which met all the selection criteria established for this review. Figure 1 outlines the search and selection process of the articles.
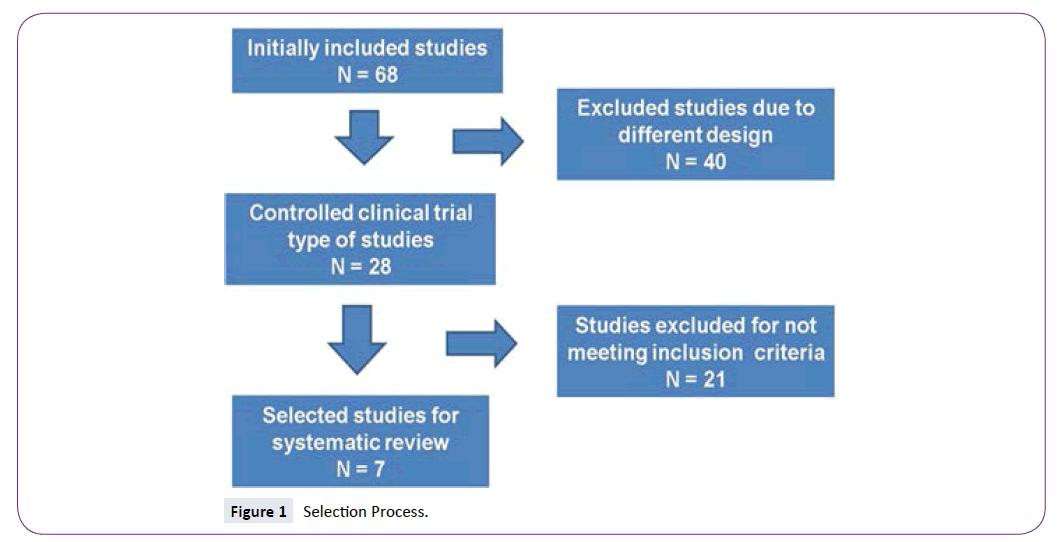
Figure 1: Selection Process.
In order to assess the quality of each of the selected articles, an individual assessment was done using the Jadad scale. This scale is based on five points consisting of whether the study is randomized. The study was protected from bias but applying the appropriate blinding procedures. The result of this individual evaluation was consistent between the three reviewers, giving a score greater than 3 in each of the studies. After running the inclusion and exclusion criteria, seven studies with the following characteristics were selected for systematic review available in Table 2. In those five studies in which cannabinoids were compared against placebo, significant differences were found in terms of decreased pain in the last week of treatment evaluation for each of the studies (p=0.02), while two studies found no statistical significant differences (p=0.5008). The results of individual trials are available in Table 3. The most common side effect of cannabinoids included: dizziness, drowsiness, dry mouth and impairments in balance. However, they were mild, not disabling, nor they negatively impacted the quality of life of patients. Tables 4 and 5 highlights the reported adverse effects in each of the studies.
| Article |
Jadad Score |
Oromucosal 9- tetrahidrocannabiol/cannabidiol for neuropathic pain: Pain associated with Multiple Sclerosis: An uncontrolled, open label 2 year extension trial
David J. Rog. BMBS,Turo J. Nurmikko, PHD, Carolyn
Clinical Theapeutics/Vol 29. N* 9, 2007[22] |
3 |
Does the cannabinoid dronabinol reduce central pain in Multiple Sclerosis? Randomized double blind plecebo controlled crossover trial
Kristina B Svendsen
BMJ 2004; 329:253. [23] |
5 |
Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis
David Rog, TuroNurmikko
Neurology 2005;65:812-819. [24] |
4 |
Analegis effect of the synthetic Cannabinoid CT-3 on chronic neuropathic pain
Matthias Klarst, KahlidSalim, Sumner Burstein
JAMA, October 1, 2003-vol. 290, No 13. [25] |
4 |
Safety, tolerability, and efficacy of orally administered cannabinoids in MS
J killestein, MD; E.L.J. Hoogervorst, MD; M. Reif, PHD;
Neurology 2002; 58: 1404-1407. [26] |
4 |
Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up
J P Zajicek, H P Sanders
J Neurol Neurosurgery Psychiatry 2005;76:1664-1669. [27] |
5 |
Double-Blind, Placebo-Controlled Trial of Lamotrigine in Combination with Other Medications for Neuropathic Pain
Marianne Silver, David Blum, Joanne Grainger, Anne E. Hammer, Steve Quessy.Journal of Pain and Symptom Management Vol. 34 No. 4 October 2007. [28] |
4 |
Table 2 Assessment of study quality by the Jadad scale.
| Article |
Design |
Sample Size |
Drugs and doses used |
Measurement scale |
| Oromucosal 9- tetrahidrocannabiol/cannabidiol for neuropathic pain: Pain associated with Multiple Sclerosis: An uncontrolled, open label 2 year extension trial David J. Rog. BMBS, Turo J. Nurmikko, PHD, Carolyn Clinical Theapeutics/Vol 29. N* 9, 2007.[21] |
Open-label uncontrolled
open-ended |
Crossover study
66 patients Controlled study
64 patients19 Open study 63 patients
indefinite time |
9- THC/cannabindiol
(27mg/ml; 25 mg/ml) |
Part One: Neuropathic pain scale Part Two: 11-point numerical scale (NRS) |
| Does the cannabinoid dronabinol reduce central pain in Multiple Sclerosis? Randomized double blind plecebo controlled crossover trial Kristina B Svendsen BMJ 2004; 329:253. [23] |
Crossover study, randomized double-blind placebo-controlled |
24 patients |
Dronabinol 2.5mg/day with increase in dose to 10mg/day Placebo |
Numerical scale 11 points
(NRS) |
| Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis David Rog, TuroNurmikko Neurology 2005;65:812-819. [24] |
Randomized double-blind placebo-controlled |
66 patients
34 patients
received
cannabinoid
32 patients
received placebo |
9- THC 2.7mg to 130mg Cannabidiol 2.5mg to 120mg |
Numerical scale 11 points
(NRS) |
| Analegis effect of the synthetic Cannabinoid CT-3 on chronic neuropathic pain Matthias Klarst, KahlidSalim, Sumner Burstein JAMA, October 1, 2003-vol. 290, No 13. [25] |
Clinical randomized, double blind placebo-controlled |
21 patients (8 women and 13 men) between 29-65 years |
Dimetillheptiltetrahydrocannabinol acid CT3 4 capsules 10 mg/day for 2 to 7 days and then 2 capsules a day for 3 days placebo |
Visual Analogue Scale
(VAS) |
| Safety, tolerability, and efficacy of orally administered cannabinoids in MS J killestein, MD; E.L.J. Hoogervorst, MD; M. Reif, PHD; Neurology 2002; 58: 1404-1407. [26] |
Randomized clinical trial with double blind placebo-controlled |
16 patients with progressive MS: 10 MS and 6 with secondary progressive MS primary |
THC and Cannabis sativa plant extract 2.5mg the first 2 weeks 25mg c/12h and increased 5 mg c/12h for 2 weeks |
Visual Analogue Scale
(VAS) |
| Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up J P Zajicek, H P Sanders J Neurol Neurosurgery Psychiatry 2005;76:1664-1669. [27] |
Randomized placebo-controlled study in two phases: first 15 weeks and followed for 12 months |
630 patients with MS 97% with progressive disease 49% outpatients |
THC 2.5mg: 1.25mg CBD and 5% other cannabinoids with maximum dose 25mg/day |
Ashworth
Scale |
Double-Blind, Placebo-Controlled Trial of Lamotrigine in Combination with Other Medications for Neuropathic Pain Marianne Silver, David Blum, Joanne Grainger, Anne E. Hammer, Steve Quessy.
Journal of Pain and Symptom Management Vol. 34 No. 4 October 2007. [28] |
Randomized placebo-controlled |
220 patients were administered 111 Lamotrigine 109 Placebo |
Lamotrigine 200, 300, or 400mg/day Placebo |
Numerical scale 11 points
(NRS) Pain scale neuropathic |
Table 3 Features selected studies.
| Article |
Study groups |
Baseline |
Last week of Treatment |
Difference |
P value |
| Oromucosal 9- tetrahidrocannabiol/cannabidiol for neuropathic pain: Pain associated with Multiple Sclerosis: An uncontrolled, open label 2 year extension trial David J. Rog. BMBS, Turo J. Nurmikko, PHD, Carolyn Clinical Theapeutics/Vol 29. N* 9, 2007. [21] |
THC: CBD |
6.6 ± 1.6 |
3.8 ± 2.1 |
-2.6 |
0.5008 |
| Placebo |
6.4 ± 1.7 |
5 ± 2.1 |
1.4 |
| Does the cannabinoid dronabinol reduce central pain in Multiple Sclerosis? Randomized double blind plecebo controlled crossover trial Kristina B Svendsen BMJ 2004; 329:253. [23] |
Dronabinol |
|
4.0(2.3- 6) |
-0.6 |
0.02 |
| Placebo |
|
5.0(4-6.4) |
|
|
| Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis David Rog, TuroNurmikko Neurology 2005;65:812-819. [24] |
THC: CBD |
6.58 (6-7.15) |
3.85 (3.13- 4.58) |
-1.25 (-2.11a-0.39) |
0.005 |
| Placebo |
6.37 (5.7-6.9) |
4.96 (4.19-5.72) |
|
|
| Analegis effect of the synthetic Cannabinoid CT-3 on chronic neuropathic pain Matthias Klarst, KahlidSalim, Sumner Burstein JAMA, October 1, 2003-vol. 290, No 13. [25] |
CTS/Placebo |
|
3 h |
8 h |
3 h |
8 h |
3 h |
8 h |
| Week 2-1 |
|
13.76 |
23.38 |
14.16 |
29.15 |
0.02 |
0.2 |
| Week 5 to 4 |
|
12.98 |
14.82 |
|
|
|
1 |
| Placebo/CTS |
|
|
|
|
|
|
|
| Week 2-1 |
|
13.11 |
11.39 |
21.43 |
10.43 |
0.02 |
0.21 |
| Week 5 to 4 |
|
22.14 |
14.48 |
|
|
|
|
| Safety, tolerability, and efficacy of orally administered cannabinoids in MS J killestein, MD; E.L.J. Hoogervorst, MD; M. Reif, PHD; Neurology 2002; 58: 1404-1407. [26] |
THC |
|
9.2 |
|
0.01 |
Sativa Extract
Placebo |
|
7.1 |
|
0.02 |
| Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up J P Zajicek, H P Sanders J Neurol Neurosurgery Psychiatry 2005;76:1664-1669. [27] |
THC |
|
|
1.82 |
0.04 |
| Extract |
|
|
0.10 (7.25) |
| Placebo |
|
|
-0.23 (7.87) |
0.01adjusted |
Double-Blind, Placebo-Controlled Trial of Lamotrigine in Combination with Other Medications for Neuropathic Pain Marianne Silver, David Blum, Joanne Grainger, Anne E. Hammer, Steve Quessy.
Journal of Pain and Symptom Management Vol. 34 No. 4 October 2007. [28] |
Lamotrigine |
1.36 |
2.08 |
|
0.67 |
| Placebo |
1.36 |
1.90 |
|
Table 4 Statistical analysis of clinical tri als.
| Article |
Adverse Effects (AE) |
| Oromucosal 9- tetrahidrocannabiol/cannabidiol for neuropathic pain: Pain associated with Multiple Sclerosis: An uncontrolled, open label 2 year extension trial David J. Rog. BMBS, Turo J. Nurmikko, PHD, Carolyn Clinical Theapeutics/Vol 29. N* 9, 2007. [21] |
EA |
THC % |
| Nausea |
17.5 |
| Diarrhea |
9.5 |
| Vomiting |
7.9 |
| Constipation |
6.3 |
| Sore Mouth |
6.3 |
| Poisoning |
11.1 |
| Peripheral edema |
9.5 |
| Fatigue |
6.3 |
| Weakness |
6.3 |
| Dizziness |
27 |
| Balance disorders |
9.5 |
| increased MS symptoms |
7.9 |
| Pharyngitis |
6.3 |
| Does the cannabinoid dronabinol reduce central pain in Multiple Sclerosis? Randomized double blind plecebo controlled crossover trial Kristina B Svendsen BMJ 2004; 329:253. [23] |
EA |
THC % |
PL% |
| izziness |
79 |
33 |
| Fatigue |
4 |
0 |
| Alerationsin balance |
25 |
4 |
| Headche |
4 |
0 |
| Migraine |
4 |
8 |
| Increased MS symptoms |
4 |
8 |
Myalgia
Nausea |
25
13 |
4
17 |
| |
|
|
| Palpitations |
17 |
8 |
| |
|
|
| Euphoria |
13 |
0 |
| Anorexia |
4 |
0 |
| Decreased weight |
4 |
0 |
| Fever |
4 |
0 |
| Chills |
0 |
4 |
| Upper airway |
4 |
0 |
| infection |
4 |
4 |
| |
|
|
Table 5 Adverse effects in each study.
Discussion
Cannabis has proven ability to manage pain associated with MS. In a clinical trial conducted on humans in 2005, cannabisbased medicine delivered in the form of a sublingual spray was demonstrated to be significantly more effective than placebo at reducing pain and sleep disturbances in MS sufferers.
We found seven randomized clinical trials which met the inclusion criteria. The best designed studies that explored the response of central pain in MS were done with cannabinoids. Only one study included an alternate drug class, the antiepileptic Lamotrigine. We found no current studies comparing different central pain drug classes.
Cannabis reduces pain in MS sufferers by directly working to reduce immune response and resultant inflammation, and also reduces musculoskeletal pain caused by muscle spasms and spasticity. A scientific review published in 2007 indicated that Δ?- THC was more effective at managing MS-related pain than CBD and dronabinol, a synthetic form of THC. Several studies have indicated that Δ?-THC, CBD, and cannabichromene (CBC) can exert an antidepressant effect and pain control. The endocannabinoid system is known to play an important role in mood regulation, pain perception and subjective levels of happiness, and endocannabinoids such as anandamide are fundamental to the process.
In studies that compared different cannabinoids against placebo it was concluded that oral Dronabinol at a maximum dose of 10 mg / day worked best in reducing pain with the least side effects. Dronabinol did show a reduction in pain, including intensity, irradiance and progression by 21%, compared to placebo [24]. The pain analogue scale ranging from 0 to 10 was used to evaluate the reduction of central pain. MS patients that used Dronabinol were reported to have a decrease of 3 points in the pain analogue scale (when initial pain ranged from 3 to 10) compared with the placebo where a significant change did not occur [24].
In comparison to the study using Dronabinol, the study which used Lamotrigine for central pain treatment showed that MS patients were reported to have a decrease of one point in the pain visual analogue scale (VAS) which was not significant when compared to placebo [27].
The number of adverse effects depends on the tolerance to the past medical history of drug tolerance of each individual, and the number of days of administration. Studies have shown good tolerance and with less adverse effects during administration of cannabinoids. These medications showed fewer side effects comparing to the anticonvulsivant lamotrigine, such as dizziness, mild drowsiness, dry mouth and impairment of balance [28].
Conclusion
MS is a disabling disease of the central nervous system that affects the quality of life of in patients. Central pain is a chronic pain syndrome known to occur commonly in MS and is often sub-optimally controlled. Relatively, few randomized controlled clinical trials have been conducted in patients with central pain associated with MS. This brief review confirmed such affirmation and was able to identify the few adequate trials that show that cannabinoids appear to be efficacious in MS associated with central pain. This was established through clinically significant reduction of central pain using the visual analogue pain scale.
Dronabinol contains synthetic delta-9-tetrahydrocannabinol; the active ingredient that binds to cannabinoid receptors in the brain and peripheral nerves to inhibit the release of glutamate, which is one of the mechanisms proposed in lowering pain. The effective dose used in the studies with the lowest side effects was 10 mg daily. The only other drug that was adequately studied in MS associated with central pain was Lamotrigine. Though, there were no clear benefits, its side effects were poorly tolerated and detrimental to the patients’ quality of life. This review raises the need for prospective studies using better designs, a sufficient number of patients, and appropriate controls aimed at examining the efficacy of cannabinoids for the treatment of central pain in patients with MS.
Recommendations
The role of cannabis for MS symptoms has not been fully defined. Future research will help determine the balance of benefits and risks of cannabis and compare its effects with other treatments available to treat spasticity, pain and other MS symptoms. More randomized controlled and comparative clinical trials in MS associated central pain are needed to further data in central pain treatment. Well-designed studies with adequate sample size would be needed in order to have a better conclusion for central pain medication treatment includin Gabapentin, Pregabaline, Carbamazepine, Oxcarbamazepine, Duloxetine, Amitriptyline, and others. Although cannabinoids appear to be efficacious in MS associated central pain, their use is limited by the poor availability, the skepticism that exists among the medical professionals on their beneficial properties and the concerns regarding risks of abuse. Education and reassurance regarding good tolerability, low risk for adverse effects and clinical effectiveness will be imperative in order for cannabinoids to be incorporated into clinical use.
8011
References
- Korsakova SS (2007) ZhurnalNevrologiiiPsikhiatriiimeni, Multiple Sclerosis 4: 5056.
- Milo R, Kahana E (2010) Multiple sclerosis: geoepidemiology, genetics and the environment.Autoimmun Rev 9: A387-394.
- Noonan CW, Williamson DM, Henry JP, Indian R, Lynch SG, et al. (2010) The prevalence of multiple sclerosis in 3 US communities.Prev Chronic Dis 7: A12.
- Alonso A, Hernán MA (2008) Temporal trends in the incidence of multiple sclerosis: a systematic review.Neurology 71: 129-135.
- Turabelidze G, Schootman M, Zhu BP, Malone JL, Horowitz S, et al. (2008) Multiple sclerosis prevalence and possible lead exposure.J NeurolSci 269: 158-162.
- Costigan M, Scholz J, Woolf CJ (2009) Neuropathic pain: a maladaptive response of the nervous system to damage.Annu Rev Neurosci 32: 1-32.
- Osterberg A, Boivie J (2010) Central pain in multiple sclerosis - sensory abnormalities.Eur J Pain 14: 104-110.
- Finnerup NB (2008) A review of central neuropathic pain states.CurrOpinAnaesthesiol 21: 586-589.
- Confavreux C, Vukusic S (2006) Natural history of multiple sclerosis: a unifying concept.Brain 129: 606-616.
- Osterberg A, Boivie J, Thuomas KA (2005). Central pain in multiple sclerosis—prevalence and clinical characteristics. European Journal of Pain 9: 531-531.
- Grasso MG, Clemenzi A, Tonini A, Pace L, Casillo P, et al. (2008) Pain in multiple sclerosis: a clinical and instrumental approach.MultScler 14: 506-513.
- Ross AP (2008) Strategies for optimal disease management, adherence, and outcomes in multiple sclerosis patients.Neurology 71: S1-2.
- Cohen BA (2008) Identification, causation, alleviation, and prevention of complications (ICAP): an approach to symptom and disability management in multiple sclerosis.Neurology 71: S14-20.
- Duran M, Capella D (2005) Cannabis y cannabinoides en el tratamientodel dolor neuropático. Dolor 20.
- Solaro C, Brichetto G, Amato MP, Cocco E, Colombo B, et al. (2004) The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study.Neurology 63: 919-921.
- Confavreux C, Vukusic S (2006) Age at disability milestones in multiple sclerosis.Brain 129: 595-605.
- Fong JS, Rae-Grant A, Huang D (2008) Neurodegeneration and neuroprotective agents in multiple sclerosis.Recent Pat CNS Drug Discov 3: 153-165.
- Newland P (2008) Pain in women with relapsing-remitting multiple sclerosis and in healthy women: a comparative study.J NeurosciNurs 40: 262-268.
- BrochetB, Deloire MS, Ouallet JC, SalortE, Bonnet M, et al. (2009) Pain and quality of life in the early stages after multiple sclerosis diagnosis: a 2-year longitudinal study. The Clinical journal of pain 25: 211-217.
- Rog DJ, Nurmikko TJ, Friede T, Young CA (2007) Validation and reliability of the Neuropathic Pain Scale (NPS) in multiple sclerosis.Clin J Pain 23: 473-481.
- Rog DJ, Nurmikko TJ, Young CA (2007) Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial.ClinTher 29: 2068-2079.
- Svendsen K, Jensen T, Bach F (2004) Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomized double blind placebo controlled crossover trial. BMJ 329: 253.
- Rog DJ, Nurmikko TJ, Friede T, Young CA (2005) Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis.Neurology 65: 812-819.
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, et al. (2003) Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial.JAMA 290: 1757-1762.
- Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van Loenen AC, et al. (2002) Safety, tolerability, and efficacy of orally administered cannabinoids in MS.Neurology 58: 1404-1407.
- Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, et al. (2005) Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up.J NeurolNeurosurg Psychiatry 76: 1664-1669.
- Silver M, Blum D, Grainger J, Hammer AE, Quessy S (2007) Double-blind, placebo-controlled trial of lamotrigine in combination with other medications for neuropathic pain.J Pain Symptom Manage 34: 446-454.






