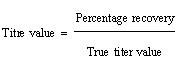Keywords
|
| Phenramine maleate; Titrant; Validation; Non aqueous; Perchloric acid |
Introduction
|
|
Spectroscopy methods
|
| Spectroscopy is the science that deals the study of interaction between electromagnetic radiation and matter [1]. It is a powerful tool to study the atomic and molecular structures and analyze the broad range of samples. The region on electromagnetic spectrum between 100 and 400 μm is included in optical spectroscopy [2]. |
|
Ultraviolet-Visible spectrophotometry
|
| UV-Visible spectrophotometry involves measurement of the amount of ultraviolet radiations absorbed by any substance in a solution. Instrument which measure the ratio or function of the ratio of the intensity of two beams of light in the U.V-Visible area are called Ultraviolet-Visible spectrophotometers [2]. |
| Beer’s law: Beer’s law says that the intensity of a beam of parallel monochromatic radiation decreases exponentially with the number of absorbing molecules. |
| Lambert’s law: Lambert’s law states that the intensity of a beam of parallel monochromatic radiation passing through a medium of homogeneous thickness decreases exponentially. A combination of these two laws yields the Beer-Lambert law [3]. |
| Beer-Lambert law: When beam of light is passed through a transparent cell containing a solution of an absorbing substance, reduction of the intensity of light may occur. Mathematical expression of Beer- Lambert law is as follows [2]. |
| A= a b c |
| Where, A=absorbance or optical density |
| a= extinction coefficient |
| b= length of radiation path through sample (cm) |
| c= concentration of solute in solution. |
| “a” and “b” are constant. |
| So “a” is directly proportional to the concentration “c” |
|
Method validation
|
| • Validation assures that a measurement method produces valid measurements [4] |
| • Results from method validation can be used to judge the Quality, consistency and reliability of analytical method can be judged by the results from method of validation [3]. |
| Method validation is the process that confirms that the analytical procedure used for a particular test is appropriate for its proposed use [5]. |
| Analytical methods are required to be validated or revalidated. |
| • Before their beginning into routine use. |
| • When the conditions are changed for which the method has been validated. |
| • When the change in method is outside the original scope of the method. |
| There are many internationally prominent organizations that offer guiding principles on method validation and other related topics [6]. |
| • American Society for Testing and Material (ASTM) |
| • Codex Committee on Methods of Analysis and Sampling (CCMAS) |
| • European Committee for Normalization (CEN) |
| • Cooperation on International Traceability in Analytical Chemistry (CITAC) |
| • European Cooperation for Accreditation (EA) |
| • Food and Agricultural Organization (FAO) |
| • United States Food and Drug Administration (FDA) |
| • International Conference on Harmonization (ICH). |
|
ICH Guidelines for analytical procedure and validation [7]
|
| These guidelines refer to the way of conducting the analysis. These should explain the steps compulsory to perform an analytical test. These may include but is not limited to: the sample, the reagents preparations and the reference standards, use of the apparatus, generation of the calibration curve, use of the formulas for the calculation, etc [3]. |
|
Non aqueous titration [8]
|
| Reactions during acid base titrations in non-aqueous solvents followed the acid base theory of Lowery Bronsted. According to this theory, proton acceptor is designated as base while proton donor is designated as acid. Substances which are insoluble in aqueous solvents undergo titration by dissolving them in organic solvents. Weak acids and bases give satisfactory result with non aqueous solutions as compared to aqueous solutions [9]. |
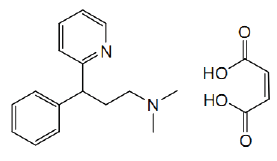
| Pheniramine Maleate (C16H20N2.C4H4O4) is an antihistamine drug and it is used to treat various hypersensitivity and allergic reactions like urticaria, hay fever and allergic conjunctivitis and also used to treat nausea, vomiting and motion sickness [10]. It also possesses anticholinergic and sedative properties as it causes sedation and may use as sleeping pills. |
| Chemically it is N,N-dimethyl-3-phenyl-3-(2-spyridyl) propylamine hydrogen maleate. Pheniramine Maleate (PM) is available in combination with other medications. PM is an important chemical as it has various therapeutic effects so its presence in every pharmaceutical formulation must be known. Therefore there should a simple and cost effective method for the determination of PM in pharmaceutical formulations (Figure 1). |
Methods and Result
|
|
Instrumentation
|
| For spectral and absorbance measurements PG double beam UV/ visible spectrophotometer was used with 2 nm band width having quartz cell of 1 cm. |
|
Chemicals
|
| Standardization of Pheniramine Maleate was done by official method of US Pharmacopoeia of volume 30 and 2007 edition. Sample was found to be 99.1%. FTIR of standard Pheniramine Maleate was also obtained and shown below. |
| Titration of Weak Bases by Non Aqueous Titration |
| Following points must be considered: - |
| 1. Titrant |
| 2. Solvent |
| 3. Preparation and standardization of 0.1N (HClO4) |
| 4. Practical examples of weak bases along with indicators. |
| 5. Typical example of assay of weakly basic substance e.g. ephedrine HCl. |
| Titrant used: 0.1N to 0.05N HClO4 solution was used in either glacial acetic acid or dioxane solution. |
|
Preliminary solubility studies of drugs
|
| Solubility of Pheniramine Maleate was checked in different solvents by taking 1gm of Pheniramine Maleate and dissolved it into 10 ml of methanol, water, 0.1N NaOH and 0.1N HCl. It was found that Pheniramine Maleate was very soluble in water, 0.1N NaOH and 0.1 N HCl. Drug is soluble in 10 parts of water so for UV spectrophotometry methanol was selected as common solvent. |
|
Preparation of stock and standards for linearity
|
| 1 mg/ml stock solutions of Pheniramine Maleate were prepared by dissolving it in solubilizing agent. These stock solutions were then diluted to 5-25 μg/ml in methanol to obtain standard solutions for calibration curve. |
|
Preparation of sample stock solution of injection
|
| Pheniramine Maleate was poured into a small beaker from 10 injections. From this beaker, almost 10 mg of the active ingredient was measured and transferred it into a 50 ml volumetric flask. Water was then added into it. It is then mixed and sonicated for 30 minutes. The same solvent was used to fill up the flask. 1ml of this solution was then poured into flask of 10 ml and Q.S water was added into it. Mixture was then filtered. |
|
Preparation of standard stock solution of raw material
|
| Drug was measured accurately equivalent to 10 mg of Pheniramine Maleate in a 50 ml volumetric flask. It was then mixed and sonicated with water for 30 minutes and then make up the volume of flask with the same solvent. Now take a flask of 10 ml and add 1ml of the prepared solution and water was used to fill up the flask. |
|
Determination of λmax’s
|
| Wavelength of maximum absorption of Pheniramine Maleate was obtained by preparation of dilutions of the standard drug solution. Methanol was used as diluents. 200-400 nm UV was used to determine the λmax’s of 20 μg/ml of drug. Maximum absorption of Pheniramine Maleate was obtained at 265 nm. The overlain spectra indicating λmax of drugs shown in Figure 2. |
|
Validation of UV/visible spectrophotometric methods Linearity and range
|
| Standard stock solution was used to prepare the further dilutions of 5, 10, 15, 20 and 25 μg/ml of Pheniramine Maleate. Water was used to fill up the volume of 10 ml of volumetric flasks. Absorbance verses concentration calibration curves of each solution was plotted after absorbance determination of solutions. Regression line equations and Correlation coefficient were determined also at 265 nm. |
|
Precision
|
| Inter and intraday precision study was performed. Drug concentration was calculated after one hour in intraday study while in inter day study drug concentration was calculated in three different days and %RSD were calculated. |
|
Accuracy
|
| Recovery study was performed at different concentrations (80%, 100%, 120%) according to ICH norms to confirm the accuracy of this method and the values were measured at all wavelengths for Pheniramine Maleate. The procedure was repeated thrice times and it was confirmed that results were within the required range when data was manipulated statistically so it is clear that this method is very accurate for quantitative estimation of Pheniramine Maleate in injection dosage forms. |
|
Limits of detections and limit of quantification
|
| The lowest amount of analyze in the sample that may be detected is known as LOD or Limit of detection. However the lowly amount of analyses in the sample that can be quantitatively determined by suitable precision and accuracy method is known as LOQ or Limit of Quantification. LOQ was determined by LOQ-10s/m while LOD was determined by LOD-3.3 s/m. Where s is the standard deviation and m is the slope of the related calibration curve. LOD and LOQ of Pheniramine Maleate were calculated using calibration curve as 3.3 σ/S respectively, where S is the slope of the calibration curve and σ is the standard deviation of response |
Results and Discussion
|
|
Linearity and range
|
| The linearity of Pheniramine Maleate were found to be in the range of 5-25 μg/ml with 0.996 for Pheniramine Maleate 265 nm , the calibration data with %RSD for both the methods and calibration curves. |
|
Precision
|
| %RSD found for the simultaneous equation method in the range of 1.005-1.78 for Pheniramine Maleate. |
|
Accuracy
|
| Accuracy of the methods was confirmed by doing recovery studies from marketed formulation at three concentration levels of standard addition. |
|
Limit of detection and of quantification limit
|
| The limit of detection found to be 0.517 for Pheniramine Maleate, respectively, the limit of quantification found to be 1.724 for Pheniramine Maleate, respectively (Figures 3 and 4) (Tables 1-4). |
|
General procedure
|
| Standardization of 0.1 mole perchloric acid: About 0.35 mg of potassium hydrogen phthalate (previously powdered lightly, dried at 120°C for 2 hours) was weighed accurately into clean and dry titration jar. It was dissolved in 50 ml of glacial acetic acid. About 0.1 ml of crystal violet solution (0.5 % w/v in anhydrous glacial acetic acid) was added. It was titrated with 0.1N per chloric acid until violet color changes to emerald green. Blank determination was performed out for necessary correction. |
| The titration was performed in duplicate. |
| One ml of 0.1N HClO4 is equivalent to 0.02042 g of potassium hydrogen phthalate (C8H5KO4) |
 |
| Where W is weight of potassium hydrogen phthalate in g and B.R. is burette reading in ml. |
| Quantitative determination of Pheniramine Maleate raw: About 0.1 g of Pheniramine Maleate test sample was weighted accurately into a clean and dried titration jar. It was dissolved in 35 ml. of anhydrous glacial acetic acid. It was titrated with 0.1N perchloric acid potentiometrically. Blank determination was also carried out for necessary correction. |
| One ml of 0.1N perchloric acid is equivalent to 17.82 mg of Pheniramine Maleate |
| % Pheniramine Meleate on the dried basis was calculated as below |
| B.R. x N x 17.82 x 100 |
| % assay = 0.1 x W |
| Where B.R. is burette reading in ml at the titrated end point. N is actual normality of 0.1 N perchloric acid. W is weight of the sample taken in g. |
| Determination of Pheniramine Maleate: The objective of this work was to determine accurately the content of Pheniramine Maleate. The assay of PM (on the dried basis) of various batches of Pheniramine Maleate test sample was analyzed using the above method. It was in the range of 98.01% to 101.5% (Tables 5). |
|
Analytical method validation
|
| The method precision was checked after analyzing six different preparations of homogeneous test sample of Pheniramine Maleate. |
| Linearity: For the establishment of method linearity ,five different weights of Pheniramine Maleate test samples corresponding to 20 % ,40 %, 60 % , 80 % and 100 % of the about weight (0.1 g) were taken and analyzed for percentage of Pheniramine Maleate content. The results are in Table 6. |
|
Accuracy and recovery
|
| Accuracy was determined at five different levels i.e., 20%, 40%, 60%, 80% and 100% of the nominal concentration (0.1 g). The titration was conducted in triplicate at each level and the titre value was recorded. The tire value obtained in linearity study was considered as true value during the calculation of percentage (%) recovery. The percentage recovery is calculated using following equation. |
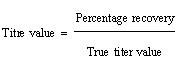 |
| The percentage range recovery of Pheniramine Maleate was in 99.2 to 99.7%. It confirms the accuracy of the proposed method (Table7). |
|
Determination of pheniramine maleate injection
|
| The objective of this work was to determine accurately the content of Pheniramine Maleate in injection. The assay of P M of various batches of Pheniramine Maleate injection test sample was analyzed using the above method. It was in the range of 98.01% to 101.5% injection contain 25 mg/ml Pheniramine Maleate. |
|
Procedure
|
| Take randomly 10 injection contain Pheniramine Maleate 25 mg/ ml, accurately measure the 4 ml solution of injection and mix in 30 ml chloroform and mix it thoroughly for 15 minutes, then start to heat for evaporation of chloroform, after full evaporation of chloroform mix acetic acid 20 ml and add few drops of naphthalene benzene and start to titrate till green color appear (Table 8). |
Conclusion
|
| Two simple, rapid, accurate and precise and most economical analytical methods were developed and validated. These two methods are more advantageous when compared to other published methods. The reported methods suffer from such draw backs as high cost, multiple steps and also several clean-up steps (HPLC). They are time consuming and often poorly reproducible, some require toxic organic solvents. Any method chosen for routine analysis should be reasonably simple, used materials should be readily available in the laboratory or readily obtainable, and require a minimum amount of equipment. These objectives have been fulfilled by the two procedures developed. The accuracy, reproducibility, simplicity and cost-effectiveness of the methods suggest their application in the quality control laboratories where the modern and expensive instruments are not available. |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
 |
 |
 |
 |
| Table 5 |
Table 6 |
Table 7 |
Table 8 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|