Keywords
Leptospirosis; Anicteric; Thrombocytopenia; Pancreatitis; Necrosis; Renal failure
Introduction
Leptospirosis was first mentioned in 1812 by Larrey and is commonly known as the "yellow fever". It is the most frequent zoonosis in the world caused by the pathogenic spirochetes from leptospira family and is characterized by a broad-spectrum of clinical manifestation varying from inapparent infection to fulminant fatal disease [1,2].
It affects both humans and animals especially, rodents. This disease is seen frequently in certain occupational groups like veterinarians, agriculture workers, slaughterhouse employees and sewage workers. Leptospirosis mainly affects liver and kidney. Rarely, other organs such as lung, heart, gallbladder, brain, and ophthalmic tissues are involved, mainly due to vasculitis [3,4].
Association of necrotizing pancreatitis with leptospirosis has been rarely reported [5-7].
Case Report
A previously healthy 35 year old male, farmer by occupation, presented to the emergency department with chief complaints of high grade fever with chills and rigors but no rash for the last 3 days. There was no history of cough, breathlessness, pain abdomen, vomiting, hemoptysis or haematemesis, altered sensorium or burning micturition. There was no history of any addiction or previous hospitalization. On examination, he was conscious, oriented, febrile with temperature of 101.6Vol. 6 No. 3:4, blood pressure of 124/76 mm Hg, pulse rate of 100/min low in volume, regular, respiratory rate 18/min with no pallor, icterus, cyanosis or clubbing. Respiratory, cardiovascular and neurological systems examination was normal. Arterial blood gas analysis was also normal. The investigations revealed hemoglobin 10.8 g/ dL (reference range: 12-16 g/dL), total leukocyte count 14,600/ mm3(reference range: 4,000-11,000/mm3), detailed leukocyte count: neutrophils 74% (reference range: 40-56%), lymphocytes 20% (reference range: 20-40%) and eosinophils 1% (reference range: 0-5%), and platelet count 78,000/mm3 (reference range: 150,000-450,000/mm3). Blood urea was 48mg/dL (reference range: 14-50 mg/dL), serum creatinine 1.4 mg/dL (reference range: 0.5-1.4 mg/dL), serum Na3 140 mEq/L (reference range: 135-145 mEq/L), K+ 4.6 mEq/L (reference range: 3.5-5.0 mEq/L), serum Ca2+ 8.2 mg/dL (reference range: 8.5-10.2 mg/dL) and blood glucose was 83 mg/dL (reference range: 70-110 mg/dL). Serum bilirubin was 1.9 mg/dL (reference range: 0.3-1.3 mg/dL) with direct 1.2 mg/dL (reference range: 0.1-0.4 mg/dL), transaminases were SGPT 46 U/L and SGOT 50 U/L (reference range: 8-40 U/L and 10-38 U/L, respectively), alkaline phosphatase was 133U/L (reference range: 13-100 U/L), serum triglyceride 124 mg/dL (reference range: 70-140 mg/dL) and serum albumin was 2.8 g/ dL (reference range: 3.5-5.5 g/dL). Malarial parasite quantitative buffy coat (MPQBC) test for Plasmodium vivax malaria, histidinerich, protein based immunochromatographic card test for Plasmodium falciparum malaria as well as peripheral blood smear examination were negative. Typhidot was negative for Salmonella typhi. Serological tests for the herpes simplex virus (HSV), dengue and NS1 antigen assay, hepatitis A, B, E, and the human immunode?ciency virus (HIV) were also negative. Chest X-ray and electrocardiography were normal. In view of the persistently thrombocytopenia, Leptospira IgM Elisa was undertaken on the second day of admission which was found to be negative (5 Panbio units). IgG negative and a Leptospira microagglutination test (MAT) was negative (at 1/20, L. Icterohemorrhagiae).
Microscopic Agglutination Test
Microscopic agglutination test (MAT) was performed with a panel of 20 serovars as shown in the Table 1. Two fold serial dilutions of serum sample were made with 0.01M phosphate buffered saline (pH 7.2) starting from 1:25. The diluted serum samples were incubated with equal volume of live cultures for 2 h at room temperature with suspensions of live leptospires. As per standard protocol, the end point was determined as highest dilution of serum showing 50% reduction in the number of free moving leptospires [8].
| Serogroup |
Serovar |
Strain |
| Australis |
lora |
Lora |
| Autumnalis |
autumnalis |
Akiyami |
| Icterohaemorrhagiae |
copenhageni |
Wijnberg |
| Louisiana |
lanka |
Le740 |
| Semaranga |
patoc |
Patoc 1 |
| Icterohaemorrhagiae |
lai |
Lai |
| Djasiman |
djasiman |
Djasiman |
| Australis |
bratislava |
Jez Bratislava |
| Sejroe |
wolfii |
3705 |
| Sejroe |
hardjo |
Hardjoprajitno |
| Pomona |
pomona |
Pomona |
| Javanica |
menoni |
Kerala |
| Celledoni |
celledoni |
Celledoni |
| Louisiana |
louisiana |
LSU1945 |
| Pyrogenes |
alexis |
HS616 |
| Autumnalis |
bulgarica |
Nikolaevo |
| Cynopteri |
cynopteri |
3522C |
| Grippotyphosa |
ratnapura |
Wumalaseena |
| Andamana |
Andaman |
Ch11 |
| Australis |
australis |
Ballico |
Table 1: List of Serovars used for microscopic agglutination test.
The cut off titre was considered as 1:100, with 50% reduction in free moving leptospires as established earlier improving outcome in patients with severe leptospirosis [9].
Leptospira IgM ELISA
Detection of IgM antibodies to Leptospira species was determined using a commercially available Leptospira IgM ELISA (Panbio Pty., Ltd., Queensland, Australia). The assay was performed according to the manufacturer’s instructions. Briefly, test sera, cutoff calibrator, and positive and negative control sera were diluted 1:100 in serum diluent, and 100 μL added to Leptospira antigen-coated microwells and incubated for 30 minutes at 37°C. After washing with phosphate-buffered saline containing 0.05% Tween 20, 100 μL of HRP conjugated anti-human IgM was added and incubated for another 30 minutes at 37°C. After further washing, 100 μL of tetramethylbenzidine substrate was added and incubated at room temperature for 10 minutes, after which the reaction was stopped with 100 μL of 1 M phosphoric acid. The absorbance of each well was read at a wavelength of 450 nm with a Bio-Tek ELX 808 plate reader (Bio-Tek Instruments, Winooski, VT). The results were expressed as Panbio units calculated by the ratio of sample absorbance to the mean cut off absorbance multiplied by 10. The recommended cut-off for a positive result is a value of ≥ 11 Panbio units, and is interpreted by the manufacturer to indicate recent infection of leptospirosis. The sensitivity and specificity of IgM ELISA on paired sera was reported to be ranging from 90.8- 100% and 55.1- 98% [10,11].
Patient was started on injection ceftriaxone 1 g 12 hourly, antipyretics and intravenous fluids. On 3rd day of hospital stay, he complained acute onset pain in abdomen localized to epigastrium and umbilical region and decrease in urine output was noticed (700 ml/24 hours) with a systolic blood pressure of 82 mmHg. On examination, abdomen was soft, but tenderness was present in the epigastrium. Bowels sounds were 4-5/min, no free fluid or organomegaly was noted. Ionotropic support was started and an erect x-ray abdomen was done which was normal. The serum amylase was 1040 U/L (reference range: 10- 200 U/L) and serum lipase was 302 U/L (reference range: 10-80 U/L), platelet count 74,000/mm3 (reference range: 150,000- 450,000/mm3), blood urea was 54 mg/dL (reference range: 14-50 mg/dL), serum creatinine 1.5 mg/dL which rose to 5.6 mg/dl on day 4 of admission (reference range: 0.5-1.4 mg/dL) alongwith with persistent oliguria. Ultrasonography of abdomen revealed a bulky pancreas, no gallstones, ascites or splenomegaly was seen. A contrast-enhanced computed tomography (CT) scan of the abdomen was done which was suggestive of diffuse pancreatitis with modified CT severity index of 6 as shown in Figure 1.
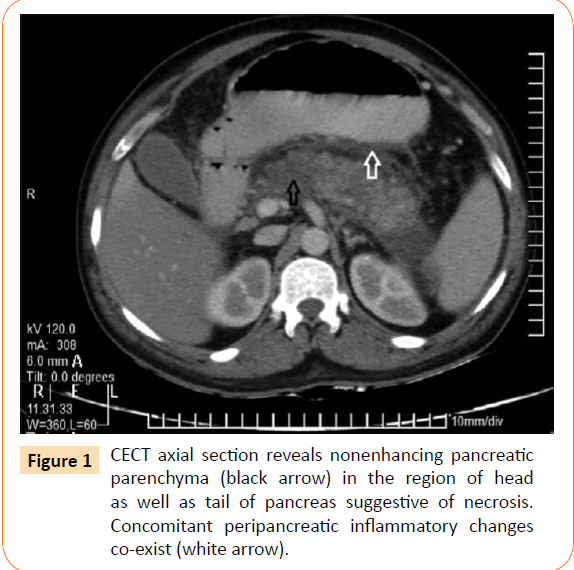
Figure 1: CECT axial section reveals nonenhancing pancreatic parenchyma (black arrow) in the region of head as well as tail of pancreas suggestive of necrosis. Concomitant peripancreatic inflammatory changes co-exist (white arrow).
In view of the persistently thrombocytopenia, Leptospira IgM Elisa was undertaken on the 7th day of admission which was found to be positive. IgG negative and a Leptospira MAT was positive (at 1/200, L. icterohemorrhagiae).
A diagnosis of Leptospira associated acute pancreatitis was made.
Patient was managed conservatively with intravenous fluids, analgesics and imipenem-cilastin 500mg thrice daily which was continued for 10 days along with supportive therapy. Ionotropic support was gradually tapered off after 36 hours. Sustained lowefficiency dialysis (SLED) was initiated on day 4 of admission with a blood flow rate of 100 ml/min and 5 sessions of SLED were given subsequently. Nasojejunal feeding tube was inserted under endoscopic guidance for adequate caloric intake. Five days after the treatment, body temperature decreased to 99.6°C, amylase levels decreased to normal value 142 U/L and platelet count increased to 160,000/mm3. Bilirubin levels, liver function tests, and creatinine level slowly returned to normal within 11 days of hospital stay. Patient recovered completely and was discharged on 15th day of admission in a stable condition (laboratory tests at the discharge time revealed WBC: 7 600/mm3, Hb: 11 g/L, total bilirubin: 0.9mg/dL, Blood urea: 38 mg/dL, Cr: 1.2 mg/dL, AST: 25 U/L, ALT: 46 U/L, LDH: 366 U/L).
Discussion
This disease occurs as two clinically recognizable syndromes: the anicteric leptospirosis (80-90% of all cases) and the remainder icteric leptospirosis [3,4].
Icteric leptospirosis is known as Weil's disease, which is characterized by hemorrhage, renal failure, and jaundice. Icteric leptospirosis is a much more severe disease than anicteric form. The clinical course is often rapidly progressing. The source of infection in humans is usually either direct or indirect contact with the urine of infected animals. These bacteria infect humans by entering through abraded skin, mucous membrane, conjunctivae. Direct transmission between humans is rare [3,4]. Leptospirosis is a common disease in rice-field workers due to prevalence of wild rats [4]. Rice field with stagnant water and humid condition is an ideal environment for leptospira. Our patient was also a farmer by occupation.
The incubation period is usually 1 to 2 weeks. Laboratory findings reveal thrombocytopenia as a common finding occurring in 40– 85% of cases. ESR and leucocyte are also elevated.
Pathogenesis of organ dysfunction is yet to be fully understood. It is thought to be related to leptospira burden, associated cytotoxic factors in the tissue especially in liver and kidney and host immune mechanism especially in lungs [12-14]. An immunological basis for pathogenesis of leptospirosis including Toll Like Receptor (TLR) 2 activation is described recently [13]. TLR 2 plays a major role in the development of pulmonary and renal manifestations of leptospirosis [15]. Leptospira lipoprotein LipL32 triggers an inflammatory response in renal proximal tubule cells by activation of TLR 2 and hence nuclear factor-kappa B and mitogen-activated protein kinases [13,15]. Though it is not yet described in relation to leptospirosis, TLR may contribute to myocarditis in sepsis and may involve in the pathogenesis of acute pancreatitis [16,17]. Bacterial peptidoglycan associated lipoprotein uses the TLR2 signaling pathway to induce cardiomyocyte dysfunction and inflammatory response in mice [16]. In acute pancreatitis, increased expression and activation of TLR 2/4 has been recognized and their role in multi-organ involvement was identified [17]. Thus a similar mechanism involving TLR may explain the presentation of our patient.
A rapid, accurate method for the diagnosis of leptospirosis is important in order to start appropriate treatment. Although leptospirosis is one of the common causes of acute febrile illness with multiorgan failure in developing countries, it remains under diagnosed mainly because of protean manifestations and lack of proper diagnostic technique [18].
MAT is considered as the gold standard for serodiagnosis of leptospirosis which was also positive in our patient, but in previous studies its sensitivity was found to vary from 30% to 76%, with a specificity of 97%. [12,19]. But in combination, the sensitivity of IgM ELISA and MAT was found to increase to 70% as reported earlier by Shekathkar et al. [20]. Several recombinant proteins (rLipL32, rLipL41) have been identified to be specific to pathogenic leptospires, but with varied sensitivity and specificity in serodiagnosis. One such recombinant protein LipL32 IgG ELISA, the sensitivity and specificity was 96.2% and 90% respectively, which was comparable with Pan Bio IgM ELISA [21]. Senthilkumar et al. have used rLipL 41, the sensitivity and specificity was found to be 89.7% and 90.45% with reference to MAT [22].
Several IgM based, commercial kits are available for the diagnosis of systemic leptospirosis using broadly reactive leptospiral antigen [18]. Winslow et al., have reported Panbio kit to be highly sensitive for diagnosis of systemic leptospirosis, the sensitivity and specificity was 100% and 98% [11]. But recently Desakorn et al. [10] using the cutoff value recommended by the manufacturer (11 Panbio units), sensitivity and specificity of IgM ELISA on paired sera was reported to be 90.8% and 55.1%. A receiver operating characteristic curve was used to determine the optimal cut-off value. This was 20 Panbio units, which gave a sensitivity and specificity of 76.1% and 82.6%, respectively, on paired sera.
The diagnosis of pancreatitis was based on biochemical and radiological evidence (Figure 1). Increased serum lipase more than 3 times the upper normal value is highly specific for pancreatitis in this patient especially at a time of normal renal functions [23,24]. The other endemic pathogens which lead to multisystem involvement such as dengue hemorrhagic fever, hepatitis virus, malaria were excluded.
Though the reported incidence of pancreatitis in leptospirosis is infrequent, in reality pancreatic involvement may be more common. Under-recognition could be due to several reasons. Pancreatic involvement could be subclinical or clinically unrecognized when dramatic and rapidly dynamic alterations of clinical and biochemical parameters take place in multi-organ dysfunction in leptospirosis. Thus a clinician may find it difficult to identify each and every complication such as pancreatitis, acalculous cholecystitis, cerebral venous thrombosis and myositis [25,26].
Additionally in a clinical setup, when a patient presents with acute pancreatitis alone as in this case, leptospirosis might not be considered as an aetiology in the initial work-up because of the rarity. Later the patient may develop multi-organ failure due to leptospirosis, yet that might be attributed to the multiorgan involvement of acute pancreatitis. Ultimately, recognition of leptospirosis might get delayed compromising the optimum management.
In disease-endemic areas, acute pancreatitis should be suspected even in anicteric leptospirosis patients with appropriate epidemiologic and clinical findings and abdominal pain; conversely, leptospirosis should be considered as a possible cause of pancreatitis. It is important to note that in our patient, acute serologic results obtained on day 5 of symptoms (hospital day 2) were negative, and the diagnosis was only obtained upon repeat testing 5 days later.
Severe leptospirosis may be fatal before IgM antibody is reliably produced, and leptospiremia may be difficult to detect; negative serologic results and blood cultures (even placed into specific growth media) do not exclude the diagnosis [4,12]. Repeat serology after the first week of illness and empirical treatment prior to serologic results may be essential for improving outcome in patients with severe leptospirosis.
Hyperamylasemia can be present in leptospirosis infection due to renal impairment, so serum lipase should be preferred [27,28]. So the diagnosis of leptospira associated pancreatitis should be made on proper clinical, biochemical and radiological findings.
In management, most of the patients of leptospirosis show spontaneous recovery and do not require any specific therapy. Although, the use of antibiotics is not well proven in leptospirosis, its early initiation can shorten the course of severity and prevent the progression of mild disease [29]. Penicillin, tetracycline, ceftriaxone and doxycycline are the preferred antibiotics. A Cochrane systematic review failed to find sufficient evidence to provide clear guidelines for use of antibiotics [30]. Patients with severe leptospirosis require correction of hypovolemia, hypotension and electrolyte abnormalities.
Conclusion
This case illustrates diagnostic difficulties especially in low resource settings with farming being one of the principle occupations in our country, where leptospirosis is more common. Although Weil’s syndrome has been seen associated with majority of the complications, our case highlights the fact that anicteric leptospirosis can be delirious too. High index of suspicion can be lifesaving in such cases which are often labelled as idiopathic pancreatitis or even antibiotic induced gastritis, especially in low resource settings.
6923
References
- Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Seica A, Covic M (2003) A retrospective 5-year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol Dial Transplant 18: 1128-1134.
- Fauci, Braunwald (2004) Leptospirosis. In: Harrison’s Principles of Internal Medicine 16th edition, Dennis L Kasper, Eugene Braunwald, Stephen Hauser, Dan Longo, J Larry Jameson, Antony S Fauci. McGraw-Hill Companies 9: 988.
- Lim SM, Hoo F, Sulaiman WA, Ramachandran V, Siew-Mooi C (2014) Acute necrotising pancreatitis and acalculouscholecystitis: a rare presentation of leptospirosis. J Pak Med Assoc 64: 958-959.
- Popa D,Vasile D, Ilco A (2013) Severe acute pancreatitis - a serious complication of leptospirosis. J Med Life 6: 307-309.
- Silva AP, Burg LB, Locatelli JF, Manes J, Crispim M (2011) Leptospirosis presenting as ascending progressive leg weakness and complicating with acute pancreatitis. Braz J Infect Dis 15: 493-497.
- Adler B, Murphy AM, Locarnini SA, Faine S (1980) Detection of specific anti-leptospiralimmunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J ClinMicrobiol 11: 452-457.
- Priya CG,Bhavani K, Rathinam SR, Muthukkaruppan VR (2003) Identification and evaluation of LPS antigen for serodiagnosis of uveitis associated with leptospirosis. J Med Microbiol 52: 667-673.
- Desakorn V, Wuthiekanun V, Thanachartwet V, Sahassananda D, Chierakul W,et al. (2012) Accuracy of a Commercial IgM ELISA for the Diagnosis of Human Leptospirosis in Thailand.Am J Trop Med Hyg 86: 524–527.
- Winslow WE, Merry DJ, Pirc ML, Devine PL (1997) Evaluation of a commercial enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in diagnosis of human leptospiral infection. J ClinMicrobiol 35: 1938-1942.
- Bharti AR,Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. (2003) Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3: 757-771.
- Palaniappan RU,Ramanujam S, Chang YF (2007) Leptospirosis: pathogenesis, immunity, and diagnosis. CurrOpin Infect Dis 20: 284-292.
- Croda J,Neto AN, Brasil RA, Pagliari C, Nicodemo AC, et al. (2010) Leptospirosis pulmonary haemorrhage syndrome is associated with linear deposition of immunoglobulin and complement on the alveolar surface. ClinMicrobiol Infect 16: 593-599.
- Yang CW (2007) Leptospirosis renal disease: understanding the initiation by Toll-like receptors. Kidney Int 72: 918-925.
- Zhu X, Bagchi A, Zhao H, Kirschning CJ, Hajjar RJ, et al. (2007) Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction.Crit Care Med 35:886-892.
- Zhang X, Zhu C, Wu D, Jiang X (2010) Possible role of toll-like receptor 4 in acute pancreatitis. Pancreas 39: 819-824.
- Rathinam SR (2005) Ocular manifestations of leptospirosis. J Postgrad Med 51: 189-194.
- Cumberland P,Everard CO, Levett PN (1999) Assessment of the efficacy of an IgM-elisa and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am J Trop Med Hyg 61: 731-734.
- Shekatkar S,Acharya NS, Harish BN, Parija SC (2010) Comparison of an in-house latex agglutination test with IgM ELISA and MAT in the diagnosis of leptospirosis. Indian J Med Microbiol 28: 238-240.
- Dey S, Mohan CM, Ramadass P, Nachimuthu K (2008) Diagnosis of leptospirosis by recombinant antigen based single serum dilution ELISA. Indian J Med Res 128: 172-177.
- Senthilkumar T,Subathra M, Phil M, Ramadass P, Ramaswamy V (2008) Rapid serodiagnosis of leptospirosis by latex agglutination test and flow-through assay. Indian J Med Microbiol 26: 45-49.
- Cappell MS (2008) Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am 92: 889-923, ix-x.
- Sutton PA,Humes DJ, Purcell G, Smith JK, Whiting F, et al. (2009) The role of routine assays of serum amylase and lipase for the diagnosis of acute abdominal pain. Ann R CollSurgEngl 91: 381-384.
- Atasoyu EM, Turhan V, Unver S, Evrenkaya TR, Yildirim S (2005) A case of leptospirosis presenting with end-stage renal failure. Nephrol Dial Transplant 20: 2290-2292.
- Turhan V,Senol MG, Sonmez G, Oncul O, Cavuslu S, et al. (2006) Cerebral venous thrombosis as a complication of leptospirosis. J Infect 53: e247-249.
- Edwards CN,Evarard CO (1991) Hyperamylasemia and pancreatitis in leptospirosis. Am J Gastroenterol 86: 1665-1668.
- Cengiz K, Sahan C, Sünbül M, Leblebicio?lu H, Cüner E (2002) Acute renal failure in leptospirosis in the black-sea region in Turkey. IntUrolNephrol 33: 133-136.
- Brett-Major DM,Coldren R (2012) Antibiotics for leptospirosis. Cochrane Database Syst Rev 2: CD008264 .
- Guidugli F, Castro AA, Atallah AN (2000) Antibiotics for treating leptospirosis. Cochrane Database Syst Rev 2:CD001306.






