Key words
|
| |
| Phyllanthus acidus, disc diffusion method, antioxidant activity, zone of inhibition, brine shrimp lethality bio-assay, phytochemical screening |
| |
INTRODUCTION
|
| |
| Medicinal plants, since times immemorial, have been used in virtually all cultures as a source of medicine . |
| |
| The widespread use of herbal remedies and healthcare preparations, as those described in ancient texts such as the Vedas and the Bible, and obtained from commonly used traditional herbs and medicinal plants, has been traced to the occurrence of natural products with medicinal properties [1].The use of traditional medicine and medicinal plants in most countries, as a normative basis for the maintenance of good health, has also been widely observed [2]. Furthermore, an increasing reliance on the use of medicinal plants in industrialized societies has been traced to the extraction and development of several drugs and chemotherapeutics from these plants as well as from traditionally used rural herbal remedies [3]. The World Health Organization has estimated that 80% of the world’s population use botanical medicine for their primary health care needs [4]. In many developing countries, traditional medicine is one of the primary health care systems [5]. Different parts are widely exploited in the traditional medicine and their curative potentials are well documented [6].Large scale evaluation of the local flora exploited in traditional medicine for various biological activities is a necessary first step in the isolation and characterization of the active principle and further leading to drug development. In view of these P. acidus plant was studied exhaustively for its potential against important thirteen human pathogenic bacteria, antioxidant and cytotoxic effects. |
| |
DESCRIPTION AND MEDICINAL USE
|
| |
| P. acidus, locally named as Arbaroi in Bangladesh and gooseberry or star gooseberry in India, is an edible small yellow berries fruit in the Phyllanthaceae family. Fruits are borne in loose clusters, are pale yellow or white, waxy, crisp and juicy, and very sour, found in Bangladesh, South India, and Southeast Asian countries. The medicinal activities of Phyllanthus species are antipyretic, analgesic, antiinflammatory, antihepatotoxic and antiviral [7-10]. |
| |
| Fruits of the two well-known species, P. acidus and P. emblica, contains high contents of vitamin C and have been used for used for improving eyesight and memory and preventive action against Diabetes and relief of coughing [11]. Another species of the family, P. amarus is an important herbal medicine due to its effective antiviral activities especially toward the hepatitis B virus [12-14]. |
| |
|
Chemicals and drugs
|
| |
| DPPH (1, 1-diphenyl, 2-picrylhydrazyl), TCA (trichloroacetic acid) and ferric chloride were obtained from Sigma Chemical Co. USA; Ascorbic acid was from SD Fine Chem. Ltd. India, ammonium molybdate from Merck, Germany. |
| |
EXPERIMENTAL
|
| |
| The collected plant part was thoroughly washed with tap water, air dried, homogenized to fine powder and stored in air tight bottles. The dried powder of plant was extracted successively in methanol by cold percolation method [15]. Ten gram of dried powder was taken in 100 ml of solvent in a conical flask, plugged with cotton wool and then kept on a rotary shaker at 190-220 rpm for 24 hours, after that the extract was filtered with eight layer of muslin cloth. The filtrate was collected in a beaker. The P. acidus plant extract was concentrated by evaporating the solvent using a water bath at a temperature of 600C. The container was allowed to air tight for 72 hours. The filtrate thus obtained was concentrated by using a rotary evaporator. |
| |
|
Phytochemical analysis:,
|
| |
| The preliminary phytochemical screening of methanol extract of P.acidus was done to ascertain the presence of bioactive components using following reagents and chemicals: Alkaloids with Dragendorff’s reagent, flavonoids with the use of Mg and HCl; tannins with ferric chloride and potassium dichromate solutions and saponins with ability to produce stable foam and steroids with Libermann Burchard reagent, Carbohydrate with Benedict’s reagent and glycoside and resins were also tested. These were identified by characteristic color changes using standard procedures [16] |
| |
|
Antibacterial assay
|
| |
| In vitro antibacterial screening is generally performed by disc diffusion method [17,18,19] for primary selection of the compounds as therapeutic agent. Disc diffusion method is equally suited to screening of antibiotics or the products of plant evaluation [20] and is highly effective for rapidly growing microorganisms and the activities of the test compounds are expressed by measuring the diameter of the zone of inhibition. In this method the compounds are applied to the agar medium by using paper discs [21,22]. The method is essentially a qualitative or semi quantitative test which allows classification of microorganisms as susceptible, intermediate or resistance to the test materials as well as bacteriostatic or bactericidal activity of a compound [23].. The bacterial strains were obtained from the Microbiology Laboratory of BCSIR, Chittagong. The diameters of the zone of inhibition produced by the compounds at the concentration 400 & 800 μg /disc were compared with the standard antibiotic (Kanamycin, 30 μg/disc). The experiments were performed at four times to minimize the error. |
| |
|
Cytotoxic study
|
| |
| Brine shrimp lethality bioassay was carried out to investigate the cytoxicity of plant extracts [24]. 50mg of Artemia salina (Leach) eggs were added to a hatching chamber containing ocean sea water (75 ml). The hatching chamber was kept under an inflorescent bulb for 48h for the eggs to hatch into shrimp larvae. The matured nauplii were then used in the experiment. Test sample was applied at different concentrations and the number of viable organisms was applied was counted after 24 hours for determination of median lethal concentration (LC50) and LC90 values. DMSO was used as a solvent and also as negative control test was one in triplicate. |
| |
|
Antioxidant Potential evaluation
|
| |
| Antioxidant potentiality of P. acidus was evaluated by determining total flavonoid contents, total antioxidant capacity, DPPH radical scavenging activity, cupric reducing antioxidant capacity and reducing capacity. |
| |
|
Determination of total flavonoids content
|
| |
| Kumaran and Karunakaran method was used to determine the total flavonoids content [25]. Here quercetin used as a reference compound. 1 mg of plant extract in methanol was mixed with 1ml aluminium trichloride in Ethanol (20 mg/ml) and a drop of acetic acid, and then diluted with Ethanol to 25 ml. after 40 min the absorption was read at 415 nm. Blank samples were prepared from 1 mg of plant extract and a drop of acetic acid, and then diluted to 25 ml with ethanol. The absorption of standard quercetin solution (0.5 mg/ml) in methanol was measured under the same conditions. |
| |
|
Determination of total antioxidant capacity
|
| |
| For total antioxidant capacity assay, according to the procedure of Prieto et al. [26]. 0.1 ml of the extract (10 mg/ml) dissolved in water was combined in a eppendorf tube with 1 ml of reagent solution (0.6 M sulfuric acid, 28mM sodium phosphate and 4mM ammonium molybdate). The tubes were capped and incubated in a thermal block at 95 °C for 90 min. After cooling to room temperature, the absorbance of the aqueous solution of each was measured at 695 nm using a spectrophotometer (Shimadzu, UV-150- 02) against a blank. Ascorbic acid was used as the standard and the total antioxidant capacity is expressed as equivalents of ascorbic acid. |
| |
|
DPPH radical scavenging activity
|
| |
| DPPH radical scavenging activity was investigated according to the method of Sreejayan and Rao, [27]. Briefly to a methanolic solution of DPPH (100m M, 2.95 ml), 0.05 ml of test compounds dissolved in methanol was added at different concentration (2— 10 mg/ml). Equal amount of methanol was added to the control. The absorbance was read at 517 nm using a spectrophotometer. Ascorbic acid was used as a standard. The inhibition curve was plotted and IC50 values were calculated. |
| |
|
Cupric Reducing Antioxidant Capacity (CUPRAC)
|
| |
| Oyaizu method was followed to determine the total reducing power capacity [28]. Extracts (100-1000 Og) in 1 ml of distilled water were mixed with 2.5 ml of phosphate buffer (0.2M, pH 6.6) and 2.5 ml potassium ferricyanide [K3Fe(CN)6] (1%), and then the mixture was incubated at 500C for 30 min. Afterwards, 2.5 ml of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. Finally, 2.5 ml of upper layer solution was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 (0.1%), and the absorbance was measured at 700 nm (21). Ascorbic acid was used as a reference standard. Phosphate buffer (pH) was used as blank solution. |
| |
|
Reducing power
|
| |
| The reducing power of P. acidus extractive was determined according to the method described by [29].. Different concentrations of P. acidus extract in 1ml of distilled water was mixed with phosphate buffer (2.5ml, 0.2M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5ml, 1%). The mixture was incubated at 50°C for 20min. A portion (2.5ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3,000rpm for 10min. The upper layer of the solution (2.5ml) was mixed with distilled water (2.5ml) and FeCl3 (0.5ml, 0.1%) and the absorbance was measured at 700nm. Ascorbic acid was used as a reference standard. Phosphate buffer (pH) was used as blank solution. |
| |
RESULTS AND DISCUSSIONS
|
| |
|
Phytochemical Screening
|
| |
| The crude extract was qualitatively tested for the presence of alkaloid, flavonoid, terpenoid, tannin, saponin, steroid, carbohydrate, glycoside and resin and the results are as follows: |
| |
| The performed qualitative studies indicate the presence of glycoside, tannin and resins while absence of carbohydrate, terpenoid, steroid, alkaloid, saponin, glucoside, and flavanoid. |
| |
|
Antibacterial assay
|
| |
| Disc diffusion methods are extensively used to investigate the antibacterial activity of natural substances and plant extracts. These assays are based on the use of discs as reservoirs containing solutions of substances to be examined. The extract was used against thirteen pathogenic bacteria with the standard antibiotic kanamycin by measuring the zone of inhibition diameter and expressed in millimeter (mm) showed in table 02. |
| |
| In the antimicrobial screening, the extract showed average zone of inhibition 8 to 12 mm (Table-02). At lower concentrations (100 μg/disc and 300 μg/disc) the extract was ineffective against all (thirteen) pathogenic bacteria which is not mentioned in table. Shigella dysenteriae, Bacillus subtilis and Bacillus megaterium show moderate inhibitory effect in both concentrations (400 μg/disc and 800 μg/disc). Whereas only inhibitory activity was noticed against the growth of Salmonella typhi and Staphylococcus aureus at higher concentrations like 800 μg/disc. |
| |
|
Brine Shrimp Lethality Bioassay
|
| |
| The methanol extract of P. acidus was tested for Brine shrimp lethality bioassay using brine shrimp nauplii and DMSO as a solvent. Control was used to see whether DMSO had any effect on brine shrimp lethality. The control group of brine shrimp nauplii with and without DMSO exhibited no mortality. For the extract, the number of nauplii died and percent mortality was counted. The result is shown in the following table- |
| |
|
Total flavonoid content and antioxidant capacity
|
| |
| Total flavonoid contents and total antioxidant capacity of P. acidus extract were expressed in quercetin and ascorbic acid equivalents respectively and are presented in Table 4. The content of antioxidant in this extract under this investigation showed moderate result (551.97 mg/g GAE) and the amount of flavonoid was 24.18 mg/g quercetin equivalent. |
| |
| Different studies suggest that different types of poly phenolic compounds like flavonoids found in plants have multiple biological effects, including antioxidant activity [30] and present studies indicate the presence of polyphenolic compound in fruit part of P. acidus. Additionally, it has been determined that the antioxidant effect of plant product is mainly due to radical scavenging activity of phenolic compounds such as flavonoids, polyphenols, tannins, and phenolic terpenes. |
| |
|
DPPH radical scavenging activity
|
| |
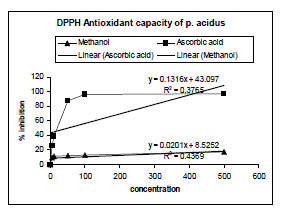
| The DPPH radical scavenging activity of P. acidus shown in Figure 01. This activity was found to slight increase with increasing concentration of the extract. The inhibition capacity of plant extract is comparatively much lower than the ascorbic acid. |
| |
| In DPPH test, which is based on the ability of DPPH, a stable free radical, to decolorize in the presence of antioxidants, is a direct and reliable method for determining radical scavenging action. Methanolic extract of P. acidus showed moderate DPPH scavenging activity. IC50 value for the plant extracts was 2063.42 Og mL−1.Ascorbic acid was chosen as the reference antioxidant for this test and IC50 value for ascorbic acid was 52.45Og mL−1. |
| |
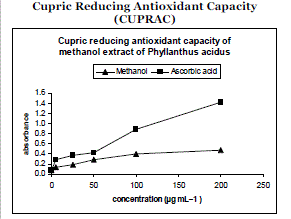
| Figure 02 represents the reductive capabilities of the plant extracts compared to Ascorbic acid which was determined using the potassium ferric cyanide reduction method. The reducing power of the extract was moderately strong while increasing dose it shows no increment. The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom. . |
| |
|
Reducing power
|
| |
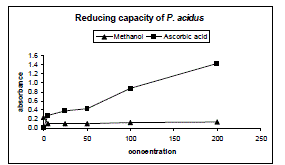
| Figure 3 represent the reductive capabilities of the plant extracts compared to Ascorbic acid which was determined using the potassium ferricyanide reduction method. The reducing power of the extracts was moderately strong while increasing dose it shows little increment. |
| |
CONCLUSION
|
| |
| From this experiment, it can be concluded that the extract showed average zone of inhibition 8 to 12 mm. As apparent from our results it can be revealed that this plant extract has narrow spectrum antimicrobial activity but it exhibited moderate to good cytotoxic effect. This indicates clearly the presence of potent bioactive principles in these crude extracts which might be very useful as antiproliferative, antitumor, pesticidal and other bioactive agents [31]. The antioxidant activity was found to slight increase with increasing concentration of the extract. The antioxidant capacity of plant extract is comparatively lowest than the ascorbic acid. Further research is needed on the determination of the correlation between the antioxidant capacity and the chemical composition of the plants. The antioxidant capacity was determined by a common used and highly accepted method. However, it was clearly noted that, there are a lot of variable results in the literature. Therefore, the higher number of antioxidant activity methods makes it necessary to use more than one method in such studies. In this study, it was concluded that the methanol extracts of the tested plant is reported to contain a myriad array of compounds, it is difficult to ascribe these observed activities to any specific group of compounds. So thoroughly investigated phytochemically and pharmacologically is necessary to exploit their medicinal and pharmaceutical potentialities. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |









