Keywords
Lactobacilli; Antibiogram; Antibacterial activity; Synergy; Growth inhibitory indices; ‘R’ values; Human pathogenic bacteria
Introduction
The three main categories of antibacterial resistances, such as multidrug resistant (MDR: which is the acquisition of nonsusceptibility to ≥3 antimicrobial agents of different categories), extensively drug resistant (XDR: means bacterial non-susceptibility to a minimum of one antimicrobial agent from all except ≤2 antimicrobial categories) and pandrug resistant (PDR: non-susceptibility to all antimicrobial agents of all categories) create a great threat to the global public health with huge economic burden, treatment failures, and increased morbidity and mortality [1-5]. This rising in the emergence of drug-resistant pathogenic bacteria led to search the nonantibiotic treatment regimens, including the probiotic lactobacilli, as new therapies to combat the bacterial antibiotic resistance and to treat the infection [6-9].
One of the important features of probiotic lactobacilliis to achieve antagonistic activity against bacterial pathogens because of their capacity to produce lactic acid and other organic acids that lower the pH in the human intestine, and to produce H2O2 and bacteriocin, thereby establishing a hostile environment for the growth and survival of various human pathogenic bacteria [10,11]. Moreover, antibiotic resistance is a required probiotic property of lactic acid bacilli, for their survival in presence of antibiotics, especially for those strains that are co-administered with antibiotics [12]; however, such resistance among the probiotic strains be essentially innate and non-transferable in nature [13]. The probiotics having innate resistance to kanamycin (K), streptomycin, gentamicin (GEN), vancomycin (VA) and nalidixic acid suggest the preventive and therapeutic administration of such strains in treating bacterial infection in the cases of co-administration with antibiotics [14-16].
Jalilsood et al. [16] evaluated the ability of Lactobacillus plantarum to form biofilm, which provided the inhibitory effect against spoilage and pathogenic bacteria. It has been reported that the L. fermentum supernatant plus bovine lactoferrin (BLF) had synergistic inhibitory activity against MRSA strains [17]. The BLF in combination with L. reuteri cell free culture supernatant demonstrated increased antibacterial activity against pathogenic bacterial strains [18]. Uraipan and Hongpattarakere [19] suggested that the combination of lactic acid bacteria and Bifidobacterium probiotic strains from human sources might be promising in the development of probiotic products in order to prevent and control the microbial infection to humans. Anal et al. [20] characterized probiotic properties of Lactobacillus acidophilus, Lactobacillus rhamnosus and Bifidobacterium bifidum from pharmaceutical probiotic sachets that on single action showed growth inhibitory property against gram-positive as well as gramnegative bacteria strains, such as Staphylococcus aureus (S. aureus), Salmonella typhi and Escherichia coli. Tomioka et al. [21] demonstrated the effect of ofloxacin-L. casei combination against Mycobacterium fortuitum infection in mice model. Bassyouni et al. [22] studied the effect L. acidophilus strains in combination with antibiotics, and found that the strains improved the antibacterial efficacy of the test antibiotics against E. coli, Salmonella typhimurium, S. aureus and Shigella flexeneri. However, no report has been made on the combined antibacterial activity of two different lactobacilli strains, at least in this part of the globe, against human pathogenic bacteria. Therefore, the current study has been undertaken to procure lactobacilli strains from probiotic sachet available in the local medical shop, to isolate and identify lactobacilli from fermented curd, and to evaluate their antibacterial activity against gram-negative human pathogenic bacteria.
Methods
Isolation and characterization of lactobacilli
The lactic acid bacteria were isolated from two fresh curd samples (commercial curd and homemade curd) following the protocol described earlier [23], and were stored in MRS agar (Hi-Media, India) slants and stabs. The non-motile grampositive non-spore forming rod-shaped bacteria, from MRS agar plates, presenting negative catalase and oxidase test results were considered as Lactobacillus, and were subjected for further phenotypic characterization: biochemical tests and sugar fermentation [23]. The curd lactobacilli thus procured were designated as S3 and DC16. Two lactobacilli strains (named as PBL2B and PBL2C strains) procured from commercially available sachets of probiotics were subjected for their identity confirmation following the methods adopted in identifying the curd lactobacilli, as mentioned above. The control strain used in the study included Lactobacillus fermentum MTCC 9748.
Antibacterial activity
Antibacterial activity of the lactobacilli procured from curd samples (S3 and DC16 strains alone and in combination) or probiotic sachet (PBL2B and PBL2C strains alone and in combination) was determined by agar overlay method [24] against the clinical isolates of E. coli and Klebsiella pneumoniae. Each of the individual Lactobacillus strains, of a pair as mentioned above, was spot inoculated alone or in combination onto MRS agar plates, with approximately 105 CFU/spot, and were incubated at 37°C for 24 h. The MRS agar plates containing the growth of lactobacilli ‘in spot form’ (≈6 mm diameter) were thereafter overlaid with Muller-Hinton agar (0.8% agar) containing a single indicator stain (E. coli or K. pneumoniae) on the individual plates and incubated at 37°C for 24 h.
Interpretation of the results
The antibacterial activity of the lactobacilli strains, alone or in combination, after 24 h incubation at 37°C, was recorded by measuring the width of clear zone (R), using the formula suggested by Carasi et al. [25] and Pisano et al. [26]:

where ‘Inhib’ denotes the diameter of clear zone around the ‘Spot’ and ‘Spot’ denotes the diameter of spot form growth of lactobacilli on MRS agar plate. Inhibition scores were considered as the no inhibition capacity with R<2 mm; low inhibition capacity when R=2-5 mm, and high inhibition capacity with R>6 mm [25,26].
The growth inhibitory indices (GIIs) were calculated using the formula, according to Mandal et al. [27]:

the synergistic and antagonistic interaction between a pair of test lactobacilli strains were defined with GIIs >0.5 and <0.5, respectively.
Antibiogram of lactobacilli
Susceptibility to 12 antibiotics, such as amikacin (AK), ciprofloxacin (CIP), cefoxitin (CX), cefotaxime (CTX), imipenem (IMP), K, trimethoprim (TR), tetracycline (TE), amoxyclav (AMC), GEN, ampicillin (AMP), vancomycin (VA) (Hi-Media, India), for the lactobacilli strains from curd and probiotic sachets was determined by disc diffusion method [28]. Single colonies of the test lactobacilli strains were inoculated into MRS broth and incubated at 37°C for 24 h. A sterilized cottonswab with test bacterial MRS broth culture was used to spread bacteria on the surface of MRS agar plate, and the antibiotic discs were placed on the surface of the agar plates. After incubation for 24 h at 37°C, the zone diameter of inhibition (ZDI) obtained around the antibiotic disc) were recorded, and isolates were categorized as sensitive (ZDI; ≥21 mm), intermediate (ZDI; 16-20 mm), or resistant (ZDI; ≤15 mm) [29,30].
Results
The curd isolates of lactobacilli, which were designated as S3 and DC16, were identified, respectively, as Lactobacillus gasseri and Lactobacillus animalis (Figure 1). The lactobacilli strains, PBL2B and PBL2C, procured from the commercial probiotic sachet were confirmed with phenotypic characterization as Lactobacillus acidophilus and Lactobacillus rhamnosus, respectively. The sugar fermentation test results of the lactobacilli strains are represented in Table 1.

Figure 1: Pure culture of L. gasseri S3 (upper portion) and L.animalis DC16 (lower portion) strains on MRS agar plate.
| Sugar |
Curd lactobacilli |
Sachet lactobacilli |
| |
DC16 |
S3 |
PBL2B |
PBL2C |
| AD |
- |
- |
- |
- |
| AR |
+ |
- |
- |
+ |
| CE |
+ |
+ |
+ |
+ |
| DE |
+ |
+ |
+ |
+ |
| LA |
+ |
+ |
- |
- |
| MA |
+ |
+ |
+ |
+ |
| MB |
+ |
w |
- |
- |
| MN |
+ |
+ |
- |
- |
| MO |
+ |
+ |
+ |
+ |
| RF |
+ |
+ |
- |
- |
| RH |
+ (gas) |
w |
W |
+ |
| SB |
+ |
+ |
- |
+ |
| SU |
+ |
+ |
W |
+ |
| TE |
+ |
+ |
+ |
+ |
| XY |
w |
w |
+ |
+ |
| Identity |
L. animalis |
L. gasseri |
L. acidophilus |
L. rhamnosus |
AD: adinitol; AR: arabinose; CE: cellobiose; DE: dextrose; LA: lactose; MA: mannose; MN: manitol; MO: Mannose; MB: melibiose; RF: raffinose; RH: rhamnose; SB: sorbitol; SU: sucrose; TE: trehalose; XY: xylose; +: positive; –: negative; w: weakly positive.
Table 1: The demographic pattern of the studied subjects.
The curd lactobacilli as well as the probiotic lactobacilli tested had excellent antibacterial activities against E. coli and K. pneumoniae clinical isolates (Figures 2 and 3). The antibacterial activity, in terms of the ‘R’ values (i.e. the diameter of clear zones) obtained due to the action of the test lactobacilli strains, alone or in combination, is represented in Figure 4. The R values for curd lactobacilli ranged 6.5-9.5 mm against the indicator bacteria, and both the probiotic lactobacilli from sachet had R value of 12 mm for E. coli, while the values ranged 8-10.5 mm for K. pneumoniae, when tested alone. In combination, the two lactobacilli strains: L. gasseri S3 (obtained from commercial curd) and L. animalis DC16 (procured from homemade curd prepared with branded milk), had increased activity against E. coli and K. pneumoniae with R values 10 mm and 10.5 mm, respectively; the PBL2B-PBL2C combined action had increased R value against E. coli (13.5 mm), only. For E. coli and K. pneumoniae, the GIIs from the PBL2B-PBL2C combination were 0.56 and 0.48, respectively, and from the S3-DC16 combination, 0.65 and 0.74, respectively.
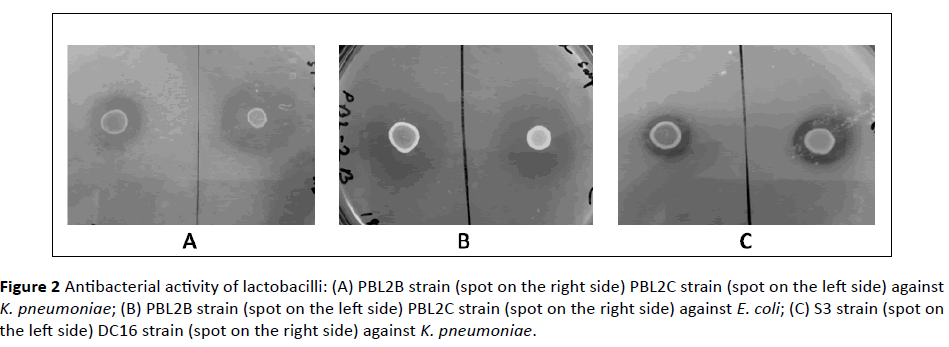
Figure 2: Antibacterial activity of lactobacilli: (A) PBL2B strain (spot on the right side) PBL2C strain (spot on the left side) against K. pneumoniae; (B) PBL2B strain (spot on the left side) PBL2C strain (spot on the right side) against E. coli; (C) S3 strain (spot on the left side) DC16 strain (spot on the right side) against K. pneumoniae.
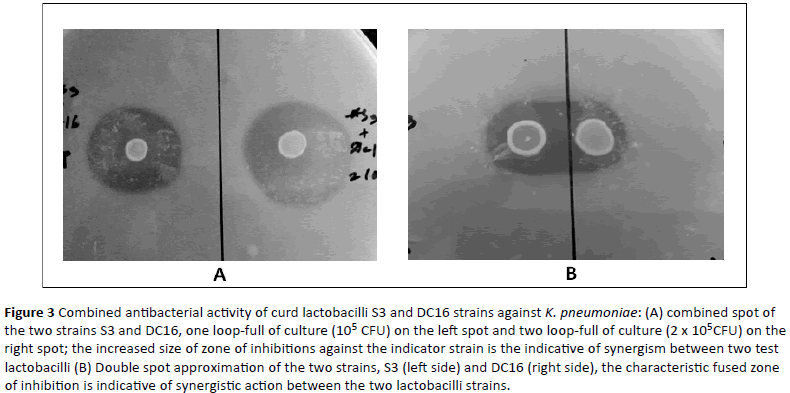
Figure 3: Combined antibacterial activity of curd lactobacilli S3 and DC16 strains against K. pneumoniae: (A) combined spot of the two strains S3 and DC16, one loop-full of culture (105 CFU) on the left spot and two loop-full of culture (2 x 105 CFU) on the right spot; the increased size of zone of inhibitions against the indicator strain is the indicative of synergism between two test lactobacilli (B) Double spot approximation of the two strains, S3 (left side) and DC16 (right side), the characteristic fused zone of inhibition is indicative of synergistic action between the two lactobacilli strains.
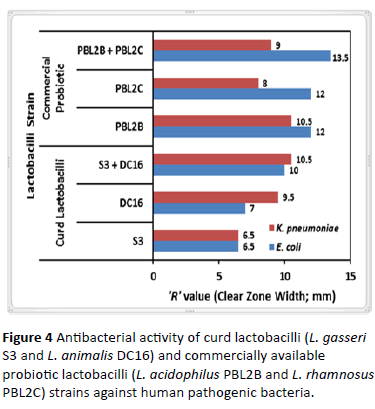
Figure 4: Antibacterial activity of curd lactobacilli (L. gasseri S3 and L. animalis DC16) and commercially available probiotic lactobacilli (L. acidophilus PBL2B and L. rhamnosus PBL2C) strains against human pathogenic bacteria.
The antibiogram test results for the lactobacilli are shown in Figure 5; the L. animalis DC16 strain showed resistance to CX and VA; moderate susceptibility to AMC, CIP and K, and sensitivity to AK, AMP, CTX, GEN, TE, TR and IMP. The L. gasseri S3 strain was resistant to CX, CIP, K, VA and TR, moderately susceptible to GEN and AK, and sensitive to AMP, AMC, CTX, TE and IMP. The L. acidophilus PBL2B strain was resistant to AK, CX, CIP, GEN, K, VA and TR, and sensitive to AMP, AMC, CTX, TE and IMP, while the L. rhamnosus PBL2C strain had resistivity to AK, CX, CIP, GEN, K, VA and TR, moderately susceptibility to AMC and TE, and sensitivity to AMP, CTX and IMP.

Figure 5: Antibiotic susceptibility test results for lactobacilli strains; PBL2B: L. acidophilus, PBL2C: L. rhamnosus, S3: L.gasseri, DC16: L. animalis. AK: amikacin, CIP: ciprofloxacin, CX: cefoxitin, CTX: cefotaxime, IPM: imipenem, K: kanamycin, TR: trimethoprim, TE: tetracycline, AMC: amoxyclav, GEN: gentamycin, AMP: ampicillin, VA: vancomycin.
Discussion
In developing countries like India, E. coli isolates causing community based infection had elevated resistance to multiple antibiotics of several kinds, and during 2008-2013, antibiotic resistance of E. coli to fluoroquinolones and extended spectrum β-lactams increased from 70%-85%, while the situation for carbapenem resistance of K. pneumoniae was with an increment from 2% (in the year 2002) to 52% (in the year 2009), as has been documented by Laxminarayan and Chaudhury [31]; the circumstances exert an impact on the outlay and the health penalty [4]. Moreover, the lifethreatening infection due to multiple antibiotic resistant bacteria is hard to treat because of the resistance to commonly as well as conventionally used antibiotics; therefore, it is essential to formulate alternative therapeutic protocol with non-antibiotic agents against the bacterial infection. Combined therapy with a mixture of classic antibiotics [32,33], or classic antibiotics and non-antibiotic antibacterial agents [27,34], has been recommended as an alternative modality for treating pathogenic infections. Zou et al. [35] reported the use of probiotics in conjunction with antibiotic in the treatment of infection with Helicobacter pylori.
Herein, we have evaluated the anti-bacterial potential of the combination of lactobacilli strains from varied sources, or from the same source against E. coli and K. pneumoniae. Davoodabadi et al. [9] reported the inhibitory activity of Lactobacillus strains of human origin and suggested their usefulness as probiotics in controlling intestinal infection with E. coli. The Lactobacillus species are usually chosen as the probiotic microorganisms because of their tolerance to low pH and bile [23], inhibitory effect against pathogenic microbes (antagonistic activity), and innate resistance to antibiotics.
Hence, lactobacilli are one such microorganisms of choice to use as the potential remedial agents, alone or in combination with one another, procured from different sources, or in combination with antibiotics; because of the therapeutic potentiality of probiotic lactobacilli against MDR bacterial infection [36], and treatment with probiotic-antibiotic combination against bacterial infection [37,38].
The antimicrobial combinations (L. casei and L. rhamnosus with AK and GEN) demonstrated synergistic actions against Pseudomonas aeruginosa, as has been reported by Aminnezhad et al. [38]. The antibiotic susceptibility test demonstrated the resistance to ampicillin, CIP and ceftriaxone for L. plantarum strain, the cell-free extract of which potentially augmented the efficacy of the test antibiotics against Salmonella infection [8]. The antimicrobial combination has been in utilization as an efficient therapeutic approach by using varied mechanisms of action; the probiotic microorganisms produce inhibitory substances, such as bacteriocin, H2O2 and organic acids, like lactic and acetic acid [11,39], in the human intestine on consumption, on the body surface on application, or in the culture medium, where they live/colonize, and inhibit the growth of pathogenic bacteria.
In this communication, which as per the knowledge and belief of the authors stands first to undertake the study of its kind at least in our part of the globe, the probiotic lactobacilli were excellent to inhibit the growth of both E. coli and K. pneumoniae (R value: 8-12 mm), and in combination such strains had synergistic effect against E. coli (GII: 0.56); for K. pneumoniae the GII was recorded as 0.48. The curd lactobacilli alone had R value of 6.5-9.5 mm, and in combination the strains showed synergism against both the indicator bacterial pathogens (GII: 0.65-0.74); moreover, S3-DC16 combination effectively had synergistic effect by modified disc approximation test (Figure 3). Such differences in the antibacterial activity of the lactobacilli are due to the strain and their source variation, difference in the target bacterial strains, and the variation in the production of antimicrobials by them, as well, which needs further study. Thus, although the probiotics, which are in current usage, are generally regarded as safe, prudence in the selection of probiotic strain(s) for patients has been considered necessary as well as urgent, and full concern for ‘risk-benefit’ ratio before prescribing is suggested, in compliance with the ‘fit for human consumption’ protocols [40].
The current research underlines the necessity of antibiotic susceptibility testing for the newly isolated lactobacilli projected for probiotic usage. The isolated curd lactobacilli (L. animalis DC16 and L. gasseri S3) strains had resistance to one or more of the antibiotics VA, CIP and K, while the commercially available lactobacilli (L. acidophilus PBL2B and L. rhamnosus PBL2C) strains showed resistance to AK, CIP, GEN, K, VA. As has been reported by Bassyouni et al. [22], the L. acidophilus-CIP combined therapy against infective gastroenteritis was effective in terms of decreasing the CIP dose and hence the CIP adverse effect on the balance of microbial flora in human intestine. Thus, the current communication suggests the usefulness of natural curd lactobacilli as well as the commercially available probiotic lactobacilli strains, alone or in combination, or in combination with some conventionally used antibiotics, as therapeutics against bacterial infection to humans. In addition, regular consumption of curd, both homemade and commercial, is required to get probiotic effect among the Indian population, since curd is an excellent source of probiotic lactobacilli; however, further studies are warranted with local curd samples regarding the current issues.
Acknowledgement
The corresponding author is grateful to the Department of Science and Technology (Ministry of Science and Technology, Government of India), for funding support of the study. (Grant number: SB/EMEQ-025/2013 dated 29-10-2013)
Conflict of interest
None
9547
References
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME,et al. (2012) Multidrugresistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18: 268-281.
- Angelis GD, D’Inzeo T, Fiori B, Spanu T, Sganga G (2014) Burden of antibiotic resistant gram negative bacterial infections: evidence and limits. J Med Microb Diagn 3: 132.
- Mandal S (2015) Can over-the-counter antibiotics coerce people for self-medication with antibiotics? Asian Pac J Trop Dis 5: S184-S186.
- Chandy SJ, Naik GS, Balaji V, Jeyaseelan V, Thomas K, et al. (2014) High cost burden and health consequences of antibiotic resistance: the price to pay.J Infect Dev Ctries 8: 1096-1102.
- Basak S, Singh P, Rajurkar M (2016) Multidrug resistant and extensively drug resistant bacteria: A Study. J Pathogens.
- Mandal S, Mandal M (2015) Coriander (Coriandrumsativum L.) essential oil: chemistry and biological activity. Asian Pacific J Trop Biomed 5: 421-428.
- Mandal MD, Mandal S (2011) Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 1: 157-160.
- Singh AP, Preet S, Rishi P (2011) Augmentation of antimicrobial activity of conventional antibiotics by cell-free extract of L. plantarum. J Antibiotics 64: 795-798.
- Davoodabadi A, Dallal MMS, Lashani E, Ebrahimi MT (2015) Antimicrobial activity of Lactobacillus spp. isolated from fecal flora of healthy breast-fed infants against diarrheagenicEscherichia coli. Jundishapur J Microbiol 8: e27852.
- Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28: 405-440.
- Jose NM, Bunt CR, Hussain MA (2015) Implications of antibiotic resistance in probiotics. Food Rev Int 31: 52-62.
- Mombelli B, Gismondo MR (2000) The use of probiotics in medical practice. Int J Antimicrob Agents 16: 531-536.
- Jose NM, Bunt CR, Hussain MA (2015) Comparison of microbiological and probiotic characteristics of lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms 3: 198-212.
- Kirtzalidou E, Pramateftaki P, Kotsou M, Kyriacou A (2011) Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe 17: 440-443.
- Tambekar DH, Bhutada SA (2010) An evaluation of probiotic potential of Lactobacillus sp. from milk of domestic animals and commercial available probiotic preparations in prevention of enteric bacterial infections. Recent Res Sci Technol 2: 82-88.
- Jalilsood T, Baradaran A, Song AAL, Foo HL, Mustafa S, et al. (2015) Inhibition of pathogenic and spoilage bacteria by a novel biofilm forming Lactobacillus isolate: a potential host for the expression of heterologous proteins. Microb Cell Fact 14: 283-288.
- Chen PW, Jheng TT, Shyu CL, Mao FC (2013) Synergistic antibacterial efficacies of the combination of bovine lactoferrin or its hydrolysate with probiotic secretion in curbing the growth of meticillin-resistant Staphylococcus aureus. J Med Microbiol 62: 1845-1851.
- Tian H, Maddox IS, Ferguson LR, Shu Q (2010) Influence of bovine lactoferrin on selected probiotic bacteria and intestinal pathogens. Biometals 23: 593-596.
- Uraipan S, Hongpattarakere T (2015) Antagonistic characteristics against food-borne pathogenic bacteria of lactic acid bacteria and bifidobacteria isolated from feces of healthy Thai infants.Jundishapur J Microbiol 8: e18264.
- Anal AKD, Singh SN, Agarwal S, Kumar R, Lawrence R (2015) Assessment of probiotic bacteria isolated from pharmaceutical probiotic sachet. Int J Life-Sciences Scientific Res 1: 94-99.
- Tomioka H, Sato K, Saito H (1990) Effect of ofloxacin combined with Lactobacillus casei against Mycobacterium fortuitum infection induced in mice, AntimicrobAgents Chemother632-636.
- Bassyouni RH, Nassar MWA, Ibrahim ZA, Ahmed MSZ (2015) The antimicrobial potential of Lactobacillus acidophilus on pathogenic bacteria causing diarrhea. Int Arabic J Antimicrob Agents 5: 2.
- Halder D, Mandal S (2015) Probiotic potentiality of curd lactobacilli. Trans Biomed 6: 8.
- Aween MM, Hassan Z, Muhialdin BJ, Noor HM, Eljamel YA (2012). Evaluation on antibacterial activity of Lactobacillus acidophilus strains isolated from honey. American J Appl Sci 9: 807-817.
- Carasi P, Diaz M, Racedo SM, Antoni GD, Urdaci MC, et al. (2014) Safety characterization and antimicrobial properties ofkefir-isolated Lactobacilluskefiri. BioMed Res Internat 208974: 1-7.
- Pisano MB, Viale S, Conti S, Fadda M, Deplano M, et al. (2014) Preliminary evaluation of probiotic properties of Lactobacillus strains isolated from Sardinian dairy products. BioMed Res Internat 286390: 1-9.
- Mandal S, Pal NK, Mandal MD (2010) Synergistic anti-Staphylococcus aureus activity of amoxicillin in combination with Emblicaofficinalis and Nymphaeodorata extracts. Asian Pacific J Trop Med 3: 711-714.
- Baeur AJ, Kirby W, Turck M (1966) Antibiotic susceptibility testing by standardized single disc method. Am J Clinl Pathol 45: 493-496.
- Liasi S, Azmi T, Hassan M, Shuhaimi M, Rosfarizan M, et al. (2009) Antimicrobial activity and antibiotic sensitivity of three isolates of lactic acid bacteria from fermented fish product Budu. Malaysian J Microbiol 5: 33-37.
- Vlkova E, Rada V, Popelarova P, Trojanová I, Killer J (2006) Antimicrobial susceptibility of bifidobacteria isolated from gastrointestinal tract of calves. Livestock Sci 105: 253-259.
- Laxminarayan R, Chaudhury RR (2016) Antibiotic resistance in India: drivers and opportunities for action. PLoS Med 13: e1001974.
- Mandal S, Pal NK, Mandal MD (2004) Synergism of ciprofloxacin and trimethoprim against Salmonella enterica serovar Typhi isolates showing reduced susceptibility to ciprofloxacin. Chemotherapy 50: 152-154.
- Mandal S, Pal NK, Chowdhury IH, Mandal MD (2009) Antibacterial activity of ciprofloxacin and trimethoprim, alone and in combination, against Vibrio cholerae O1 biotype El Tor serotype Ogawa isolates. Polish J Microbiol 58: 57-60.
- Mandal S, Mandal MD, Pal NK (2012) Enhancing chloramphenicol and trimethoprim in vitro activity by Ocimum sanctum Linn. (Lamiaceae) leaf extract against Salmonella enterica serovar Typhi. Asian Pacific J Trop Med 5: 220-224.
- Zou J, Dong J, Yu X (2009) Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter 14: 97-107.
- Jamalifar H, Rahimi H, Samadi N, Shahverdi A, Sharifian Z, et al. (2011) Antimicrobial activity of different Lactobacillus species against multi- drug resistant clinical isolates of Pseudomonas aeruginosa. Iran J Microbiol 3: 21-25.
- Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, et al. (2004) In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol 70: 518-526.
- Aminnezhad S, Kermanshahi RK, Ranjbar R (2015) Evaluation of synergistic interactions between cell-free supernatant of Lactobacillus strains and amikacin and genetamicin against Pseudomonas aeruginosa. Jundishapur J Microbiol 8: e16592.
- Dunne C, O’Mahony L, Murphy L, Thornton G, Morrissey D (2001) In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 73: 386S-392S.
- Fijan S (2014) Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int J Environ Res Public Health 11: 4745-4767.











