Research Article - (2023) Volume 15, Issue 2
Anticancerous Effect of Fruit pulp of Aegle marmelos Against Benzo[A]pyrene Induced Lung Tumours in Rats
Jyotsna Sethi1*,
Chandrajeet Kumar2 and
Arun Kumar2
1Department of Biotechnology, YBN University, Ranchi, Jharkhand, India
2Mahavir Cancer Sansthan and Research Centre, Patna, Bihar-801505, India
*Correspondence:
Jyotsna Sethi, Department of Biotechnology, YBN University, Ranchi, Jharkhand,
India,
Tel: +91-9334740800,
Email:
Received: 27-Feb-2023, Manuscript No. ijddr-23-13509;
Editor assigned: 13-Mar-2023, Pre QC No. ijddr-23-13509;
Reviewed: 16-Mar-2023, QC No. ijddr-23-13509;
Revised: 23-Mar-2023, Manuscript No. ijddr-23-13509;
Published:
30-Mar-2023
Abstract
In the recent times, the lung cancer disease burden in the population has increased manyfolds globally. Among those carcinogen- Benzo[A]pyrene is one of the known which is directly responsible for the cause. Benzo(a)pyrene has been shown to inflict damage on the lungs as well as liver. Thus, the present study has been aimed to study the anticancer activity of Aegle marmelos fruit pulp extract on Benzo [A] pyrene induced lung cancer in rats. Thirty male Charles Foster rats, 6 weeks old weighing around (150-180 g) were used for the study and were induced Benzo[A] pyrene (25 mg/Kg dissolved in Olive oil) orally in two intervals (1st day and 14th day) and were left for 3 months. After 3 months, there was development of lung tumours, which were confirmed histopathologically. Thereafter, Aegle marmelos fruit pulp extract at the dose of 250mg/Kg body weight was administered to the rats for 5 weeks. After the treatment there was significant reduction in the lung tumour size was observed in the studied rats. All the parameters were studied and their data were analysed. The haematological parameters, the biochemical parameters and the histopathological parameters were also correlated for the efficacy of the drug.
Through the entire study, it can be concluded that, fruit pulp extract of Aegle marmelos possesses anticancerous effect against Benzo [A]pyrene induced lung cancer. This drug after various trials can be recommended as therapeutic drug for lung cancer disease in the future.
Keywords
Benzo[A]pyrene induced lung model; Tumour volume; Biochemical parameters study; Histopathological study; Fruit pulp extract of Aegle marmelos; Novel drug discovery
Introduction
In state of Bihar, a large number of populations inhabit in villages
with agriculture as major professional practice. A large number of
rural families depend upon preparation of food in direct contact
with fire, burning of wood or cow dung cakes which releases lot of
smoke that results to various health related issues. The polycyclic
aromatic hydrocarbons (PAHs) are usually released from burning
of organic matters (Hyland et al. 2020). The PAHs are a group
of large family of compounds that contain aromatic rings made
up of only carbon and hydrogen atoms [1]. A large number of
PAHs have been found to be toxic, which in longer duration of
exposure causes gene alterations and leading to development
of diseases like lung cancer (Balmes 2018; Hussain et al., 2014).
The air pollution in the recent times has caused serious lung
diseases in the older and younger age group such as asthma,
bronchitis, recurrent cough and breathlessness and moreover
the chronic exposure also causes the disease of lung cancer.
Among the severe carcinogen is a Benzo(a)pyrene which are Poly
Aromatic Hydrocarbons (PAHs) generated by partial burning of
diverse organic material. Its metabolic activation occurs through
cytochrome P450 to the 7, 8-dihydrodiol-9, to epoxide i.e. the
eventual cancer causing agent which is considered for DNA
damage and inducing carcinogenesis (Kasala et al. 2015) [2].
In the Indian medicine system Ayurveda there are plethoras of
Jadi Bootis (medicinal plants) which have potent treatment for
disease of lungs. Among them, Aegle marmelos or Wood apple
has promising role against the various types of diseases such as
gastrointestinal disorders, antimicrobial, antiviral, antipyretic,
ulcer healing, diuretic, antimicrobial, anti-inflammatory,
radioprotective and chemopreventive properties. The
A.marmelos contains furocomarins, xanthotoxol, and methyl
ester of alloimperatorinm flavonoids, rutin and marmesin. The
leaves, barks, roots, fruits and seeds are extensively used by the
people of Indian subcontinent as traditional or folk medicine for
various ailments. Various parts of this tree are used for the ailment
of disease including cancer (Akhouri et al. 2020, Baliga et al. 2013
and 2010, Manandhar et al. 2018, Ramakrishna et al.2015, Bhatti et al.2013, Agrarwal et al.2010, Rahman and Parvin 2014) [3-5].
Hence, the present study deals to develop Benzo[a]pyrene
induced lung cancer model in Charles Foster rats and evaluate
the efficacy of pulp extract of A.marmelos against the lung
tumors [6].
Materials and Methods
Chemicals and reagents: Benzo[a]pyrene (C20H12) manufactured
by Sigma-Aldrich, USA, Product number B1760-100MG, (CAS
Number: 50-32-8), Lot# SLBV8459, P code: 1002545809 was
purchased from the Scientific chemical store of Patna, Bihar,
India and was provided by the Research Department of Mahavir
Cancer Sansthan and Research Centre, Patna, India. All the other
solvents and chemicals used were of analytical grade 99% [7].
Medicinal plant: As a medicinal plant, Aegle marmelos is
commonly known as Wood apple or Indian Bael was used in the
study. The Aegle marmelos fruit were procured from the local
market of Patna and were identified and certified by a botanist
Prof. Ashok Kumar Ghosh. The seeds and fibre from Aegle
marmelos fruit were removed and the pulp was extracted and
dried in incubator at 37oC. Then grinded in to fine powder and
later soaked in absolute ethanol for 48hrs and finally extracted
with absolute ethanol using Rota vapour apparatus. The dose
of the ethanolic pulp extract of Aegle marmelos was titrated to
75mg/kg body weight per day for 5 weeks after the estimation of
LD50 value [8, 9].
Ethical approval: Before the use of animals, ethical approval
was obtained from the Institutional Ethics Committee (IAEC) of
the institute through CPCSEA (GoI) with CPCSEA Registration no.
1129/bc/07/CPCSEA. The research work was approved from the
IAEC no. 2021/1B-06/10/21 dated 06/10/2021 [10].
Animals: Male Charles Foster rats were provided by the animal
house of Mahavir Cancer Sansthan and Research Centre, Patna,
India. The rats were housed in standard polypropylene cages
housing 02 rats in each case. They were randomly divided into
control and treatment groups. The room temperature was
maintained at 22 ± 2 °C for rats with 12 hours light/dark cycle and
the animals had free access to food and water ad libidum [11].
Lung tumor model development: For inducing the lung tumor,
rats were treated with Benzo[a]pyrene at the dose of 25 mg/Kg
dissolved in Olive oil orally in two intervals (1st day and 14th day) and were left for 3 months. After, 3 months the animals were
development of lung tumors which were confirmed through fine
needle aspiration and few animals were dissected for lung biopsy
for final confirmation of the lung cancer [12].
Experimental design: Thirty male Charles Foster rats, 6 weeks old
weighing (150-180 g) were divided into groups of n=6 animals in
each.
Group I- Control (n=6)
Group II- Benzo[a]pyrene treated (n=12)
Group III- Aegle marmelos treated (n=6)- Upon Benzo[a]pyrene
induced treated with Aegle marmelos ethanolic pulp extract
(250mg/kg body weight per day) for 5 weeks (Group II rats).
Rats were anaesthetized and sacrificed after the completion
of the dose. Blood samples were obtained through the orbital
puncture of the experimental rats. For biochemical test and lipid
peroxidation estimation serum were separated. Tissues of lungs
and other organs were fixed in 10% formalin for histopathological
studies [13-15].
Biochemical assay: Biochemical analysis were performed through
the serum by standard kit process (Coral crest) on (UV - Vis)
spectrophotometer (UV-10, Thermo Scientific, USA). The Liver
function test (LFT) as serum glutamate pyruvate transaminase
(SGPT) and serum glutamate oxaloacetate transaminase (SGOT)
were measured according to the method Reitman and Frankel
(1957), alkaline phosphatase (ALP) by using method of Kind and
King (1954), total bilirubin activity by method Jendrassik and Grof
(1938). The kidney function test (KFT) was analyzed through urea
by the method of Fawcett and Scott (1960); Berthelot (1859),
creatinine by the method of Bones and Tausky (1945) and uric
acid by the method of Fossati and Prencipe (1980) [16, 18].
Lipid peroxidation (LPO): Thiobarbituric acid reactive substances
(TBARS) are used as markers of LPO, was evaluated through the
double heating method (Draper and Hadley 1990) applied on
the principle of spectrophotometric measurement of colour
reproduced during the reaction to thiobarbituric acid (TBA) with
malondialdehyde (MDA). In this study, 2.5ml of 10% solution
of Trichloroacetic acid (TCA) was mixed with 0.5ml serum in a
centrifuge tube then heated in water bath for 15 minutes at 90°C.
Then after, the mixture was left for cooling at room temperature,
the mixture was further centrifuged at 3000rpm for 10 minutes.
The 2ml supernatant was mixed with 1 ml of 0.675% TBA solution
in test tube, which was again heated in water bath for 15 minutes
at 90°C. The solution was left for cooling at room temperature.
Absorbance was further measured by UV-Visible spectrophotometer (Thermo Scientific UV-10 USA) at 532nm [19-22].
Superoxide dismutase (SOD) activity: The epinephrine
technique Misra et al., (1972) was used to measure SOD activity
in the supernatant. The technique relies on measuring the rate
of epinephrine auto-oxidation inhibition by SOD contained in the
examined samples in 50 mM sodium carbonate buffer pH 10.2,
within the linear range of auto-oxidative curve and U/mg protein
units were used to express the SOD activity [23].
Catalase (CAT) activity: The catalase (CAT) activity was analysed by using the technique of Goth et al., (1991). During this
procedure serum samples were incubated in substrate containing
65 μmol/ml hydrogen peroxide in 60 mmol/l sodium-potassium phosphate buffer, pH 7.4 for 60s at 37 °C, Under these conditions
one unit of CAT decomposed in 1 μmol of hydrogen peroxide
(H2O2) per minute. Then and there 32.4 mM (NH4)2MoO4
(Ammonium molybdate) were used to stop enzymatic reaction
and the yellow complex of molybdate and hydrogen peroxide was
detected at 405 nm in comparison to a blank that contained all
the components except the enzyme [24, 25].
Histopathological study: Lung tissues were fixed into 10% formalin for 24hours. Then tissues were dehydrated through
graded series of ethanol and embedded into paraffin wax. The
4.5μm section were cut and stained by haematoxylin and eosin
for histopathological study under light microscope (Cardiff et al.,
2014) [26].
Statistical analysis: Results are presented as mean ± standard
deviation (SD) for six rats in individual groups. Total variation
represented in a set of data were analyzed through one-way
analysis of variance (ANOVA) followed by Tukey’s test with
multiple comparisons (p<0.05 was statistically considered).
Calculations were performed with the GraphPad Prism program
(GraphPad 5 Software, Inc., San Diego, USA) [27].
Results
Morbidity and Mortality: In Benzo[a]pyrene treated group,
there was mild mortality observed in the group. However, mild
sluggishness was observed at the end of the treatment. Severe
breathlessness was observed in every group which gradually
decreased on administration of Aegle marmelos.
Effect of A.marmelos on liver functional test
(LFT)
In comparison to the control group, in Benzo[a]pyrene treated
group there was significant (p<0.0001) rise in the levels of SGPT, SGOT, ALP, and total bilirubin. But, after the treatment
with the hydroxyethanolic pulp extract of A.marmelos there
were significant (p<0.0001) decrease in the serum levels of
SGPT, SGOT, ALP, and total bilirubin. The evidences suggests
that A.marmelos has protective effect against Benzo[a]pyrene
induced hepatotoxicity [28] (Table 1).
| Parameters |
Control |
Benzo [A] pyrene Treated |
A.marmelos Treated for 5 Weeks |
| |
|
|
|
| SGPT (U/mL) |
35.89 ± 3.7 |
189.23± 8.67* |
100.4 ± 7.25* |
| SGOT (U/mL) |
37.56 ± 2.8 |
210.29± 9.32* |
93.26± 1.67* |
| ALP (KA units) |
9.57 ± 1.06 |
39.87 ± 1.56** |
13.25± 1.74** |
| Bilirubin (mg/dL) |
0.95 ± 1.79 |
3.12 ± 0.5* |
1.9 ± 1.99** |
Table 1. Showing Liver function test data.
Effect of A.marmelos on Kidney functional test
(KFT)
In comparison to the control group, in Benzo[a]pyrene treated
group there was significant (p<0.0001) rise in the levels of urea,
uric acid, creatinine, and albumin. But, after the treatment with
the hydroxyethanolic pulp extract of A.marmelos there were significant (p<0.0001) decrease in the serum levels of urea,
uric acid, creatinine, and albumin. The evidences suggests that
A.marmelos has protective effect against Benzo[a]pyrene induced
nephrotoxicity [29] (Table 2).
Parameters |
Control |
Benzo [A] pyrene Treated |
A.marmelos Treated for 5 Weeks |
| |
|
|
|
| Urea (mg/dL) |
32.15 ± 2.67 |
70.18 ± 2.67** |
46.33 ± 1.78** |
| Uric acid (mg/dL) |
3.74 ± 0.84 |
17.95 ± 2.55** |
9.67 ± 2.06* |
| Creatinine (mg/dL) |
0.68 ± 0.92 |
5.27 ± 2.05* |
1.97 ± 1.28* |
Table 2. Showing Kidney function test data.
Effect of A.marmelos on Lipid peroxidation (LPO)
The serum level LPO is significantly higher (p<0.0001) in Benzo[a]
pyrene treated rats in comparison to the control group. However,
there was substantial (p<0.0001) decrease observed after the
hydroxyethanolic pulp extract of A.marmelos administration
had significant changes compared to control. This suggests the
antioxidant potential of pulp extract of A.marmelos [30] (Figure 1).
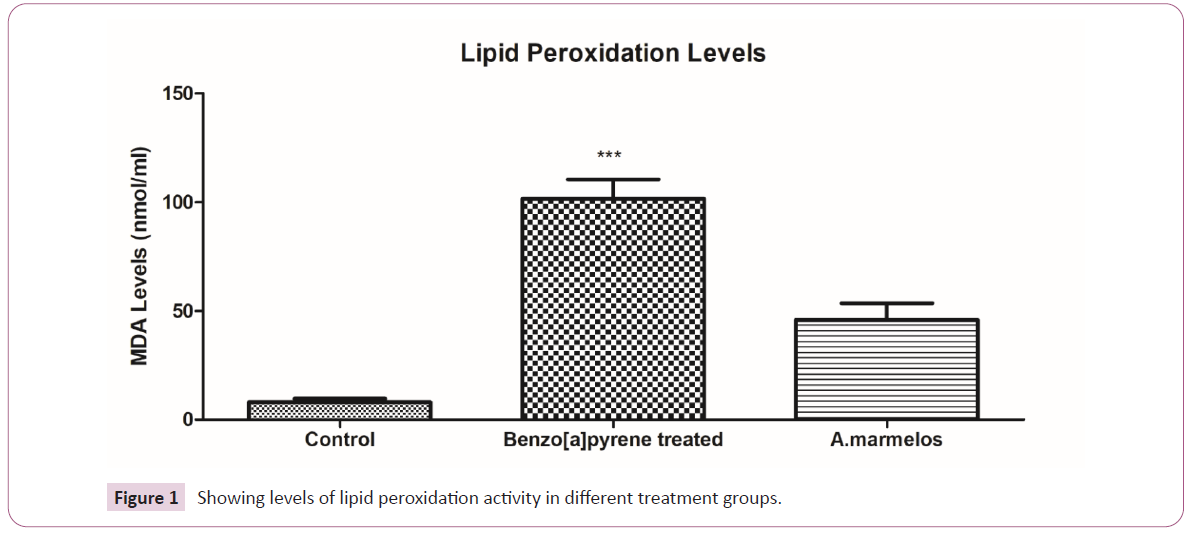
Figure 1: Showing levels of lipid peroxidation activity in different treatment groups.
Effect of A.marmelos on Superoxide Dismutase
(SOD)
The SOD activity significantly decreased (p<0.0001) in Benzo[a]
pyrene treated rats in comparison to the control group. However,
there was substantial (p<0.0001) increase observed after the
hydroxyethanolic pulp extract of A.marmelos administration
had significant changes compared to control. This suggests the
antioxidant potential of pulp extract of A.marmelos [31] (Figure 2).
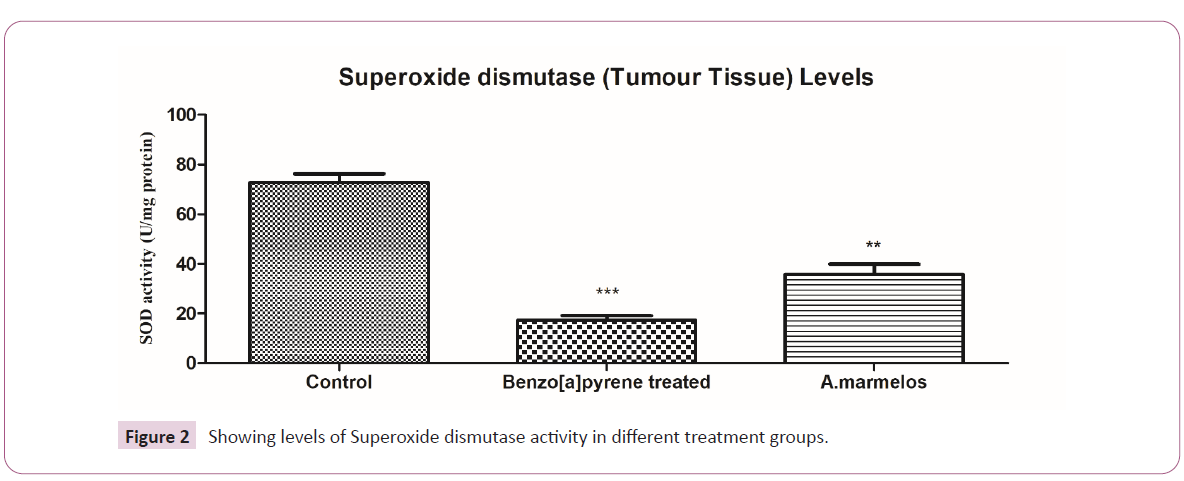
Figure 2: Showing levels of Superoxide dismutase activity in different treatment groups.
Effect of A.marmelos on Catalase (CAT) activity
The CAT activity significantly decreased (p<0.0001) in Benzo[a]
pyrene treated rats in comparison to the control group. However,
there was substantial (p<0.0001) increase observed after the
hydroxyethanolic pulp extract of A.marmelos administration
had significant changes compared to control. This suggests the antioxidant potential of pulp extract of A.marmelos [32] (Figure 3).

Figure 3: Showing levels of Catalase activity in different treatment groups.
Histopathological study
The histopathological study shows normal architecture of
alveolar sacs in the tissue of lung. The parietal and visceral layers
appear to be with normal functioning of the lung (Figure 4A). In
the Benzo[A]pyrene treated rats shows lung cells with papillary
tubulo-alveolar carcinoma (Figure 4B). But, after the treatment
with the pulp extract of A.marmelos there has been significant
reversal in the cells of lung. However, the residual disease is
mildly still persistent. (Figure 4C and 4D).
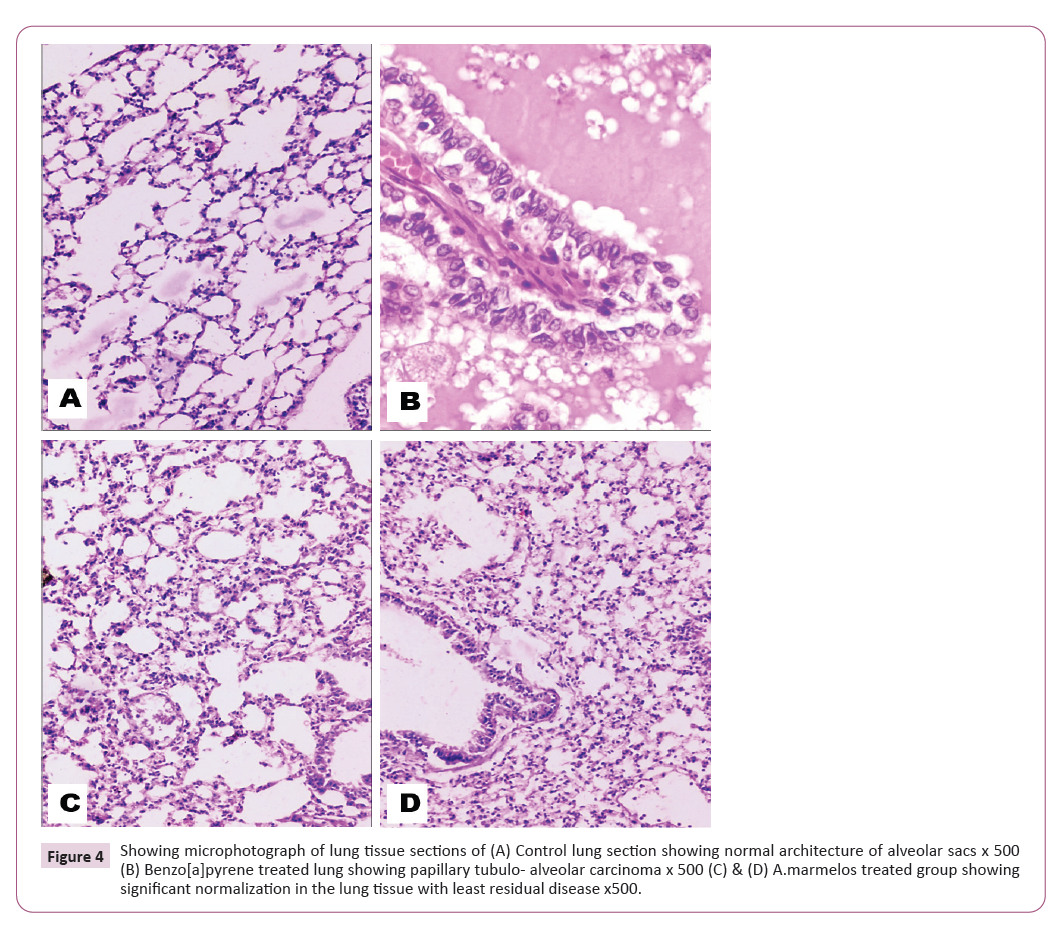
Figure 4: Showing microphotograph of lung tissue sections of (A) Control lung section showing normal architecture of alveolar sacs x 500
(B) Benzo[a]pyrene treated lung showing papillary tubulo- alveolar carcinoma x 500 (C) & (D) A.marmelos treated group showing
significant normalization in the lung tissue with least residual disease x500.
Showing microphotograph of lung tissue sections of (A) Control
lung section showing normal architecture of alveolar sacs x
500 (B) Benzo[a]pyrene treated lung showing papillary tubulo-
alveolar carcinoma x 500 (C) & (D) A.marmelos treated group
showing significant normalization in the lung tissue with least
residual disease x500 [33-36].
Discussion
Benzo[A]pyrene is the most common environmental carcinogen to which humans are exposed to it frequently. In the present study,
the lung tumor formed after the treatment with benzo[A]pyrene
in the tissue was papillary tubulo-alveolar carcinoma, which is
the prominent disease model formed in the present study. The
benzo[A]pyrene exposure usually causes activates and increases
the expression of the histone H3 lysine 9 methyltransferase.
Moreover, it also suppresses the function of the tumor suppressor
gene SOCS3 disrupting the functions of the caspase series and
furthermore activating the Akt and Erkl/2 pathways leading to
tumorigenesis in lungs. The similar model formation was made by
various research workers (Wang et al., 2020; Martin & Fry 2018;
Baltayiannis et al., 2008 Wang 2021; Gu et al., 2018; Chen et al.,
2016) [37].
In the present study, Beanzo[A]pyrene induced lung tumor model
was developed and was confirmed histopathologically, as there
was significant papillary tubulo- alveolar carcinoma formed in the
lung. But, after the treatment with A.marmelos fruit pulp, there was significant reversal in the lung cells as the normal alveolar
sacs were clearly observed. This result confirms that fruit pulp of
A.marmelos contains anti-carcinogenic activities. The fruit pulp
contains active ingredients such as marmelosin, lupeol, eugenol,
citral, cineole and limonene which have probably anti-cancer
effects [38]. Similar, A. marmelos extract effect have been reported
as anticancerous effect in various models (Akhouri et al., 2020;
Sain et al., 2014). Pynam and Dharmesh., 2018, have discovered
its potent activity as antioxidant and anti-inflammatory in
inhibition of TNF- alpha mediated tumor model. They have found
marmelosin as the potent anti-tumor compound controlling the
TNF-alpha mediated Akt signaling pathway. Agrawal et al., 2011,
have reported the anticancerous effect against the chemically
induced skin cancer in mice models [39].
Furthermore, in the present study there has been significant
decrease (p<0.05) in the antioxidant effects in Benzo[A]pyrene
treated group in comparison to the control group. There was significant decrease (p<0.05) in the superoxide dismutase
levels and catalase levels, while significant increase (p<0.05) in
the lipid peroxidation levels. But after the treatment with the
fruit pulp extract of A.marmelos, there was significant (p<0.05)
normalization in the levels of superoxide dismutase, catalases
and lipid peroxidation levels, The fruit pulp of A.marmelos
contains flavonoids, vitamin B and C, thus shows the antioxidant,
inflammatory, anti-ulcer activity. Various authors have correlated
their study with the effect of pulp extract of A.marmelos (Agrawal
et al 2011; Chaubey and Dubey 2020; Ahmad et al., 2021; Mujeeb
et al., 2018; Venthodika et al., 2021) [40].
In the present study, to observe the effect of drug side effects,
the vital organs such as liver and kidneys biochemical parameters
were evaluated, which showed significant (p<0.05) increase in
the levels of SGPT, SGOT, ALP bilirubin, urea and uric acid and
creatinine levels but after the administration of the pulp extract
of A.marmelos, there was significant (p<0.05) normalization in
the levels in the liver and kidney biochemical parameters except
SGPT, SGOT, bilirubin and creatinine levels. However, there was
significant (p<0.05) decrease in the levels in comparison to the
Benzo[A]pyrene treated group but still was higher than the
normal ranges [42]. It could be probably due to the severe damage
caused by the Benzo[A]pyrene and the anti-inflammatory activity
in the liver and kidney organs might be slower as they are the
metabolic organs. Furthermore, it is quite possible that if the dose
of the pulp extract of A.marmelos could have increased for more
duration, there could have been significant normalization in the
biochemical parameters level. However, the significant decrease
in the liver and kidney functions could be due to the presence of
flavonoids which proves to be the major active constituent having
the hepato-renal protective effect. The moderate regeneration is
the vital effect of A.marmelos. Various other researchers have found the hepato-renal protective effect of A.marmelos (Sharma
et al., 2022; Yang et al., 2020; Rajasekaran et al., 2009; Patel et al.,
2012; Baliga et al., 2011; Dwivedi et al., 2017) [42].
Conclusions
From the entire study, it can be concluded that, Benzo[A]pyrene
causes formation of lung tumors in Charles Foster rats, but there
was significant reduction in the lung cancer disease due to the
fruit pulp extract effect of the A.marmelos. Moreover, there
was significant normalization in the free radicals levels-lipid
peroxidation, superoxide dismutase and catalase activity. But, in
the studied biochemical parameters of liver function tests and
kidney function tests, there was significant normalization in the
levels of ALP, urea and uric acid but moderate decrease in the
levels of SGPT, SGOT, bilirubin and creatinine levels. The study
finally concludes that A.marmelos possesses anticancerous,
antioxidant and moderate hepato-renal protective activity.
Furthermore duration of A.marmelos fruit pulp extract could
have made more protection against liver and kidney organs.
Hence, this drug has therapeutic anticancerous property against
lung cancer.
Acknowledgements
The authors are thankful to Mahavir Cancer Sansthan and
Research Centre for providing the experimental infrastructure
and YBN University for the entire research facilities.
Conflict of Interests
The authors declare that they have no conflicts of interest.
References
- Agrawal A, Verma P, Goyal PK (2010) Chemo modulatory effects of Aegle marmelos against DMBA-induced skin tumorigenesis in Swiss albino mice. Asian Pac J Cancer Prev 11:13-14.
Indexed at, Google Scholar
- Agrawal A, Jahan S, Goyal PK (2011) Chemically induced skin carcinogenesis in mice and its prevention by Aegle marmelos fruit extract. J Environ Pathol Toxicol Oncol 30: 251-259.
Indexed at, Google Scholar, Crossref
- Ahmad W, Amir M, Ahmad A, Ali A, Wahab S et al (2021) Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities. Plants 10: 2573.
Indexed at, Google Scholar, Crossref
- Akhouri V, Kumari M, Kumar A (2020) Therapeutic effect of Aegle marmelos fruit extract against DMBA induced breast cancer in rats. Sci Rep 10:180-186.
Indexed at, Google Scholar, Crossref
- Baliga MS, Bhat HP, Pereira MM, Mathias N, Venkatesh P et al (2010) Radioprotective effects of Aegle marmelos (L.) Correa (Bael): a concise review. J Altern Complement Med 16:1109-1116.
Indexed at, Google Scholar, Crossref
- Baliga MS, Thilakchand KR, Rai MP, Rao S, Venkatesh P et al (2013) Correa (Bael) and its phytochemicals in the treatment and prevention of cancer. Integr Cancer Ther 12:187-96.
Indexed at, Google Scholar, Crossref
- Baliga MS, Bhat HP, Joseph N, Fazal F (2011) Phytochemistry and medicinal uses of the bael fruit (Aegle marmelos Correa). Food R Int. 44:1768-1775.
Indexed at, Google Scholar, Crossref
- Balmes JR, Eisen EA (2018). Household air pollution and chronic obstructive pulmonary disease. A Riddle, Wrapped in a Mystery, Inside an Enigma. Am J Respir Crit Care Med. 197:547-549.
Indexed at, Google Scholar, Crossref
- Baltayiannis G, Baltayiannis N, Tsianos EV (2008) Suppressors of cytokine signaling as tumor repressors. Silencing of SOCS3 facilitates tumor formation and growth in lung and liver. J Environ Pathol Toxicol Oncol 13:263-265.
Indexed at, Google Scholar
- Berthelot MPE (1859) Report Chim. Appl 45:28-84.
Indexed at, Google Scholar, Crossref
- Bhatti R, Singh J, Saxena AK, Suri N, Ishar MP et al (2013) Pharmacognostic standardisation and antiproliferative activity of Aegle marmelos (L.) Correa leaves in various human cancer cell lines. Indian J Pharm Sci 75:628-634.
Indexed at, Google Scholar
- Bones RW, Tausky HH (1945) Colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 158:581-91.
Indexed at, Google Scholar, Crossref
- Cardiff RD, Miller CH, Munn RJ (2014) Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc 2014:655-658.
Indexed at, Google Scholar, Crossref
- Chaubey A, Dubey AK (2020) Chemistry and Antioxidant Potential of Phytoconstituents from Aegle Marmelos Fruit-Shell. Curr Drug Metab 21: 525-533.
Indexed at, Google Scholar, Crossref
- Chen H, Lee L, Li S, Tsao G, Chiu JF et al (2016) Up regulation of glycolysis and oxidative phosphorylation in benzo[α]pyrene and arsenic-induced rat lung epithelial transformed cells. Oncotarget 7: 40674-40689.
Indexed at, Google Scholar, Crossref
- Draper HH, Hadley M (1990). Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 186: 421-431.
Indexed at, Google Scholar, Crossref
- Dwivedi J, Singh M, Sharma S (2017) Antioxidant and nephroprotective potential of Aegle marmelos leaves extract. J Herb Spices Med Plant 23: 363-377.
Indexed at, Google Scholar, Crossref
- Fawcett JK, Scott J (1960) A rapid and precise method for the determination of urea. J Clin Path. 13: 156-159.
Indexed at, Google Scholar, Crossref
- Fossati P, Prencipe L (1980) Enzymatic colorimetric method of the determination of uric acid in serum. Clin Chem 26: 227.
Indexed at, Google Scholar
- Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Int j clin chem 196: 143-151.
Indexed at, Google Scholar, Crossref
- Gu Q, Hu C, Chen N, Qu J (2018) A comparison between lung carcinoma and a subcutaneous malignant tumor induced in rats by a 3, 4-benzopyrene injection. Int j clin chem lab med 11: 3934-3942.
Indexed at, Google Scholar
- Hussain T, Al-Attas OS, Al-Daghri NM, Mohammed AA, De Rosas E et al (2014) Induction of CYP1A1, CYP1A2, CYP1B1, increased oxidative stress and inflammation in the lung and liver tissues of rats exposed to incense smoke. Mol Cell Biochem 391: 127-36.
Indexed at, Google Scholar, Crossref
- Hyland KC, Smith IK, Shukla SD, Hansbro PM, Zosky GR et al (2020) Cow Dung Biomass Smoke Exposure Increases Adherence of Respiratory Pathogen Nontypeable Haemophilus influenzae to Human Bronchial Epithelial Cells. Expo Health 12: 883-895.
Indexed at, Google Scholar, Crossref
- Jendrassik GF, Grofs BM (1938)Quantitative colorimetric determination of bilirubin in serum or plasma. Clin Chem Acta 27: 79.
Indexed at, Google Scholar
- Kasala ER, Bodduluru LN, Barua CC, Sriram CS, Gogoi R et al (2015) Benzo(a)pyrene induced lung cancer: Role of dietary phytochemicals line. Pharmacol Rep 67:996-1009.
Indexed at, Google Scholar, Crossref
- Kind PRH, King EJ (1954) Determination of Alkaline Phosphatase activity in serum. J Clin Path 7: 322.
Indexed at, Google Scholar, Crossref
- Manandhar B, Paudel KR, Sharma B, Karki R (2018) Phytochemical profile and pharmacological activity of Aegle marmelos Linn. J Integr Med. 16: 153-163.
Indexed at, Google Scholar, Crossref
- Martin EM, Fry RC (2018) Environmental influences on the epigenome: exposure associated DNA methylation in human populations. Annu Rev Public Health. 39: 309-333.
Indexed at, Google Scholar, Crossref
- Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247: 3170-3175.
Indexed at, Google Scholar
- Mujeeb F, Khan AF, Bajpai P, Pathak N (2018) Phytochemical Study of Aegle marmelos: Chromatographic Elucidation of Polyphenolics and Assessment of Antioxidant and Cytotoxic Potential. Pharmacogn Mag 13: 791-800.
Indexed at, Google Scholar, Crossref
- Patel PK, Sahu J, Sahu L, Prajapati NK, Dubey BK (2012) Aegle marmelos: A review on its medicinal properties. Int J Pharm Phyto Pharmacol Res 1: 332-341.
Indexed at, Google Scholar, Crossref
- Pynam H, Dharmesh SM (2018) Antioxidant and anti-inflammatory properties of marmelosin from Bael (Aegle marmelos L.); Inhibition of TNF-α mediated inflammatory/tumor markers. Biomed Pharmacother 106: 98-108.
Indexed at, Google Scholar, Crossref
- Rahman S, Parvin R (2014). Therapeutic potential of Aegle marmelos (L.). Asian Pac J Trop Dis 4: 71-77.
Indexed at, Google Scholar, Crossref
- Rajasekaran C, Kalaivani T, Ramya S, Jayakumararaj R (2009) Studies on hepatoprotective activity of ethanolic extracts of fruit pulp of Aegle marmelos (L.). Corr J Pharm Res 2: 1419-1423.
Indexed at, Google Scholar, Crossref
- Ramakrishna YG, Savithri K, Kist M, Devaraj SN (2015) Aegle marmelos fruit extract attenuates Helicobacter pylori Lipopolysaccharide induced oxidative stress in Sprague Dawley rats. BMC 15: 375.
Indexed at, Google Scholar, Crossref
- Reitman S, Frankel SA (1957) Colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 56-63.
Indexed at, Google Scholar, Crossref
- Sain S, Naoghare PK, Devi SS, Daiwile A, Krishnamurthi K et al (2014) Beta caryophyllene and caryophyllene oxide, isolated from Aegle marmelos, as the potent anti-inflammatory agents against lymphoma and neuroblastoma cells. Med Chem 13: 45-55.
Indexed at, Google Scholar, Crossref
- Sharma D, Mir NA, Biswas A, Deo C (2022) Performance enhancing, immunomodulatory, anti-hyperlipidaemic, and antimicrobial properties of bael (Aegle marmelos) leaf powder in broiler chicken. Trop Anim Health Prod 54: 56.
Indexed at, Google Scholar, Crossref
- Venthodika A, Chhikara N, Mann S, Garg MK, Sofi SA et al (2021). Bioactive compounds of Aegle marmelos L., medicinal values and its food applications. Phytother Res 35: 1887-1907.
Indexed at, Google Scholar, Crossref
- Wang Z (2021) Mechanisms of the synergistic lung tumorigenic effect of arsenic and benzo(a)pyrene combined-exposure. Semin Cancer Biol 76: 156-162.
Indexed at, Google Scholar, Crossref
- Wang Z, Yang P, Xie J, Lin HP, Kumagai K et al (2020) Arsenic and benzo[a]pyrene co-exposure acts synergistically in inducing cancer stem cell-like property and tumorigenesis by epigenetically down-regulating SOCS3 expression. Environ Int 137: 105-560.
Indexed at, Google Scholar, Crossref
- Yang P, Xie J, Li Y, Lin HP, Fenske W et al (2020) Deubiquitinase USP7-mediated MCL-1 up-regulation enhances Arsenic and Benzo(a)pyrene co-exposure-induced Cancer Stem Cell-like property and Tumorigenesis. Theranostics 10: 9050-9065.
Indexed at, Google Scholar, Crossref
Citation: Sethi J, Kumar C, Kumar A (2023)
Anticancerous Effect of Fruit pulp of Aegle
marmelos against Benzo[A] pyrene Induced
Lung Tumours in Rats. Int J Drug Dev Res J,
Vol. 15 No. 3: 1001.










