Keywords
Atrial fibrillation, Anticoagulation, Hypertension
Stroke risk in the elderly
Older individuals with AF are at a higher risk of stroke compared to younger patients. The 2014 American Heart Association/ American College of Cardiology/Heart Rhythm Society (AHA/ ACC/HRS) guidelines [5] recommend using the CHA2DS2-VASc (Congestive heart failure (CHF), Hypertension, Age ≥75 years, Diabetes, Stroke/Transient ischemic attack (TIA), Vascular disease, Age 65-74, Sex category (female)) score (Table 1) to assess stroke risk (Class I; Level of evidence B). These guidelines recommend oral anticoagulant (OAC) therapy for AF patients with a CHA2DS2- VASc >2 (Class I recommendation) with either warfarin (Level of evidence A), dabigatran, rivaroxaban or apixaban (Level of evidence: B) (Table 2) and no antithrombotic therapy, OAC or aspirin (ASA) in patients with a CHA2DS2-VASc score of 1.
| CHADS2 |
Score |
CHA2DS2-VASc |
Score |
HAS-BLED |
Score |
Congestive heart failure/
LV dysfunction |
1 |
Congestive heart failure/
LV dysfunction |
1 |
Hypertension |
1 |
| Hypertension |
1 |
Hypertension |
1 |
Abnormal renal/ liver function |
1 or 2 |
| Age ≥ 75 years of age |
1 |
Age ≥ 75 years of age |
2 |
Stroke |
1 |
| Diabetes Mellitus |
1 |
Diabetes Mellitus |
1 |
Bleeding tendency |
1 |
| Stroke/ TIA |
2 |
Stroke/ TIA |
2 |
Labile INRs |
1 |
| |
|
Vascular disease (MI, PVD, or aortic plaque) |
1 |
Age (> 65, frail) |
1 |
| |
|
Age 65-74 |
1 |
Drugs (NSAIDs/ ASA) or alcohol abuse |
1 |
| |
|
Sex category (female) |
1 |
|
|
| CHADS2: Congestive heart failure, Hypertension, Age > 75, Diabetes Mellitus, Stroke/ TIA; CHA2DS2- VASc : Congestive heart failure, Hypertension, Age > 75, Diabetes Mellitus, Stroke/ TIA, Vascular disease, Age 65-74, Sex category (female); HAS-BLED: Hypertension, Abnormal renal or liver function, Stroke, history of Bleeding, Labile INRs, Elderly, Drug or alcohol abuse; LV: Left Ventricular dysfunction; TIA: Transient Ischemic Attack; MI: Myocardial Infarction; PVD: Peripheral Vascular Disease; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs |
Table 1: CHADS2, CHA 2DS2-VASc, and HAS-BLED scores [8].
| CHA2DS2VAScscore |
ACCF/AHA/HRS |
ACCP |
CCS |
| 0 |
No antithrombotic therapy |
No antithrombotic therapy or ASA or ASA + Clopidogrel. Preferably no therapy. |
No anti thrombotic therapy |
| 1 |
No antithrombotic therapy or ASA or OAC based on assessment of bleeding risk, and patient preference. |
OAC recommended over no therapy/ ASA/ ASA+ Clopidogrel. For patients who are unsuitable for or choose not to take OAC (for reasons other than a high risk for major bleeding) combination therapy with ASA + Clopidogrel is recommended over ASA alone. |
Oral anticoagulation therapy. One of the newer anticoagulants recommended in preference to warfarin.
ASA may be considered based on individual risk/ benefit assessment. |
| >2 |
OAC |
OAC, with
Dabigatran 150 mg bid preferred over warfarin therapy |
Oral anticoagulation therapy. One of the newer anticoagulants is recommended in preference to warfarin (except in patients already on warfarin, with stable INR’s and no bleeding complications). |
| CHADS2 :Congestive heart failure, Hypertension, Age > 75, Diabetes Mellitus, Stroke/ TIA. The updated ESC guidelines recommend using the CHA2DS2-VASc score to evaluate the risk for stroke in AF patients; ACCF/ AHA/ HRS: American College of Cardiology/ American Heart Association/ Heart Rhythm Society; ACCP: American College of Chest Physicians; CCS: Canadian Cardiovascular Society; OAC: Oral Anticoagulation |
Table 2: ACCF/AHA/HRS [5], CCS [6], and ACCP [7] guidelines based on CHA2DS2 VASc scores.
The CHA2DS2-VASc score appropriately designates age ≥ 75 years as a major risk factor (2 points), equivalent to a history of previous stroke/TIA, making anticoagulant therapy a Class I indication for this group of patients even in the absence of other risk factors (Figure 1).
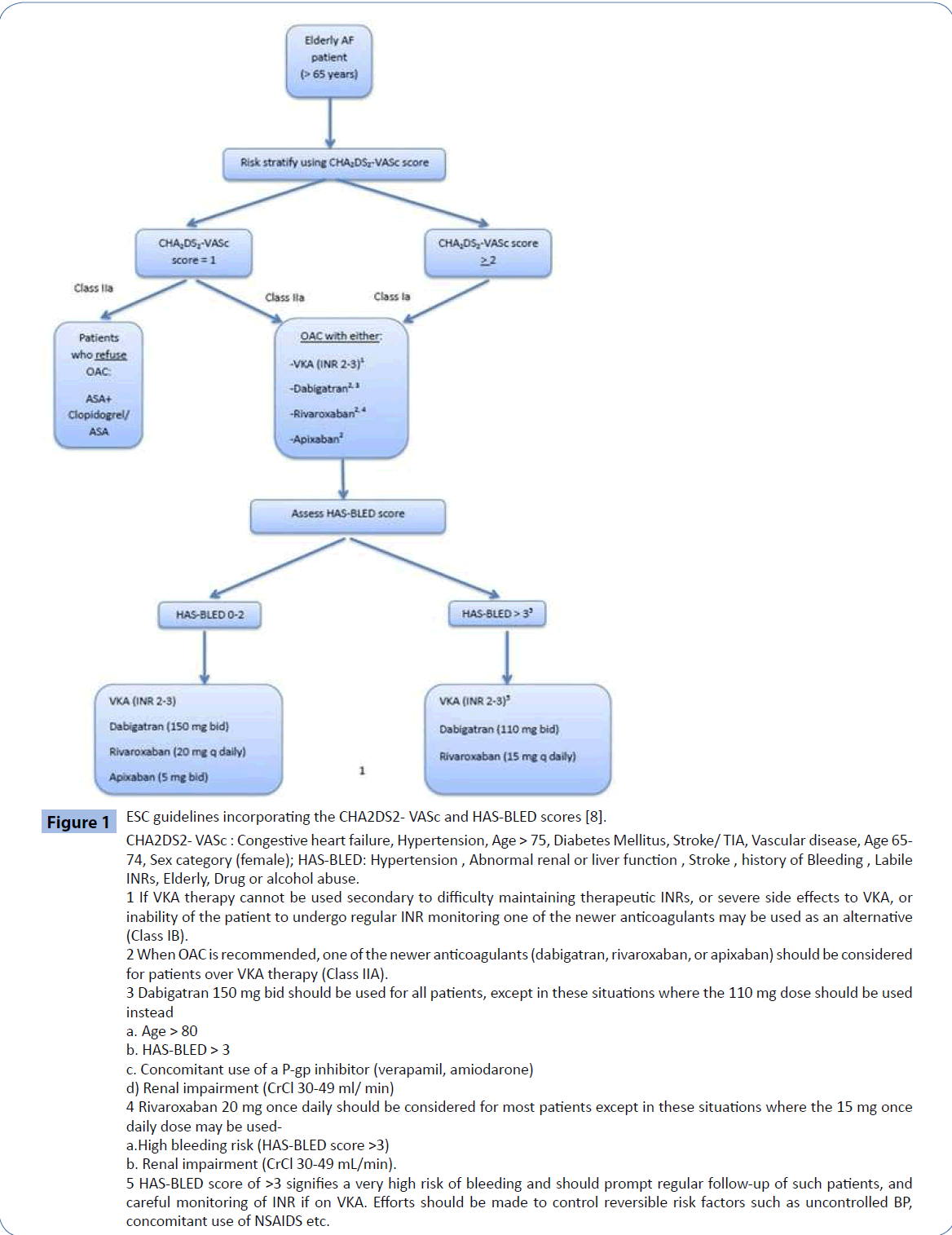
Figure 1: ESC guidelines incorporating the CHA2DS2- VASc and HAS-BLED scores [8]. CHA2DS2- VASc : Congestive heart failure, Hypertension, Age > 75, Diabetes Mellitus, Stroke/ TIA, Vascular disease, Age 65- 74, Sex category (female); HAS-BLED: Hypertension , Abnormal renal or liver function , Stroke , history of Bleeding , Labile INRs, Elderly, Drug or alcohol abuse. 1 If VKA therapy cannot be used secondary to difficulty maintaining therapeutic INRs, or severe side effects to VKA, or inability of the patient to undergo regular INR monitoring one of the newer anticoagulants may be used as an alternative (Class IB). 2 When OAC is recommended, one of the newer anticoagulants (dabigatran, rivaroxaban, or apixaban) should be considered for patients over VKA therapy (Class IIA). 3 Dabigatran 150 mg bid should be used for all patients, except in these situations where the 110 mg dose should be used instead a. Age > 80 b. HAS-BLED > 3 c. Concomitant use of a P-gp inhibitor (verapamil, amiodarone) d) Renal impairment (CrCl 30-49 ml/ min) 4 Rivaroxaban 20 mg once daily should be considered for most patients except in these situations where the 15 mg once daily dose may be useda. High bleeding risk (HAS-BLED score >3) b. Renal impairment (CrCl 30-49 mL/min). 5 HAS-BLED score of >3 signifies a very high risk of bleeding and should prompt regular follow-up of such patients, and careful monitoring of INR if on VKA. Efforts should be made to control reversible risk factors such as uncontrolled BP, concomitant use of NSAIDS etc.
How are we doing in terms of thromboprophylaxis in the elderly AF population?
Only about 46.5% of patients at a high risk for stroke are prescribed OAC therapy [9,10]. Reasons for under-prescription of OAC therapy include concerns about an increased risk of bleeding in the elderly, food-drug and drug-drug interactions, increased sensitivity to warfarin in the elderly, variable dose response, difficulty maintaining the target international normalized ratio (INR), cognitive and physical impairments in the elderly, and physicians’ fears about the risk of falling in the geriatric population. There have been several studies to estimate the risk/ benefit ratio of treating elderly AF patients with OAC, including the Birmingham Atrial Fibrillation Treatment of the Aged (BAFTA) study [9], which randomized individuals aged ≥75 years of age to aspirin or warfarin based on physician discretion. Stroke/systemic embolism (SE) occurred at a rate of 1.8%/year in warfarin-treated patients versus 3.8%/year in the aspirin group (hazard ratio [HR] 0.48; 95% confidence interval (CI), 0.28-0.80), with no difference in the rates of major hemorrhage (1.9 vs. 2.2%, respectively).
The target range for INR to reduce the risk of stroke/SE is 2-3, with levels below and above this range associated with an increase in thromboembolism and major bleeding respectively [11]. There have been several studies evaluating the use of lower targets for anticoagulation in elderly patients. A nested case-control study of the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) [12] population found that the risk of thromboembolism increased at INR levels <1.8 (odds ratio (OR), 3.72) while the odds of intracranial hemorrhage (ICH) increased at INR values above 3.5. A recent study by Cafolla et al. [13] enrolled 116 AF patients >80 years of age and divided them equally into two groups based on target INRs (target INR of 2.0 (1.5 to 2.5) versus 2.5 (2.0 to 3.0). Patients in the lower INR target group showed a significantly increased time in therapeutic range (TTR) compared to the latter group (72.59% versus 64.43%, P<0.01). Patients in the higher INR target group experienced more bleeding and thrombotic events than the lower target group. The lower incidence of thromboembolic events in the group with the lower target INR could potentially be explained by the lower INR target mean deviation, and better tolerance of the drug in this group of patients. However, it should be kept in mind that the benefit of using a lower INR targets was seen in fragile, elderly (>80 years of age) patients with previously documented unstable INRs and frequent INR values above 5.
Challenges associated with warfarin therapy in the elderly
Quality of anticoagulation
In patients treated with warfarin for AF, improved survival was seen in those patients with a TTR>40%. However, the risk for stroke in these patients decreased significantly only when the TTR was >70% [14]. Maintaining an adequate INR can be challenging in elderly patients owing to various factors. Older age is associated with an exaggerated response to OAC, with lower doses of warfarin providing adequate and sometimes excess anticoagulation. Moreover, the prevalence of polypharmacy rises with age, and this can cause large fluctuations in INR. However, age alone should not be a deterrent to maintaining good quality anticoagulation [15]. Frequent and close anticoagulation monitoring of dose-adjusted warfarin is crucial to prevent bleeding events in elderly patients on warfarin.
Other factors
Declining renal function is one of the most important factors affecting the pharmacokinetics of OACs in the elderly. Elderly patients with a normal serum creatinine can still have significantly impaired renal function. The AHA/ACC/HRS guidelines recommend evaluating renal function prior to initiation of the newer OACs, and at least once a year. The 2012 CCS focused update recommends that all AF patients should have an annual assessment of their renal function and undergo regular assessments for drug/dose changes based on their estimated glomerular filtration rate (eGFR). Patients with chronic kidney disease and a creatinine clearance (CrCl)>30 ml/min should be treated with antithrombotic therapy based on their CHA2DS2- VASc score as in patients with normal renal function. Those with a CrCl of 15-30 ml/min should receive antithrombotic therapy based on their CHA2DS2-VASc score, but the preferred agent is warfarin. Warfarin is recommended for patients with end stage renal disease (CrCl <15 ml/min) or on hemodialysis (Class IIa recommendation). Multiple co-morbidities, polypharmacy, risk for falls, and declining cognitive function can further complicate the management of elderly AF patients on anticoagulation.
Bleeding with warfarin therapy
What is the actual risk?
The risk for bleeding with warfarin therapy in patients >80 years of age has been estimated to range anywhere from 1.63% to 13.1% per year [15,16]. The risk for hemorrhage increases with an INR ≥ 4.0, older age and in the first 90 days of therapy (Figure 2). Recently, Gomes et al. [17] studied the rates of major bleeding in 125,195 AF patients on warfarin, >66 years of age. The overall risk of bleeding in this study was 3.8% per year, somewhat higher than that seen in clinical trials (1-3%). The risk of major bleeding in patients >75 years was 4.6% versus 2.9% in those <75 years of age.
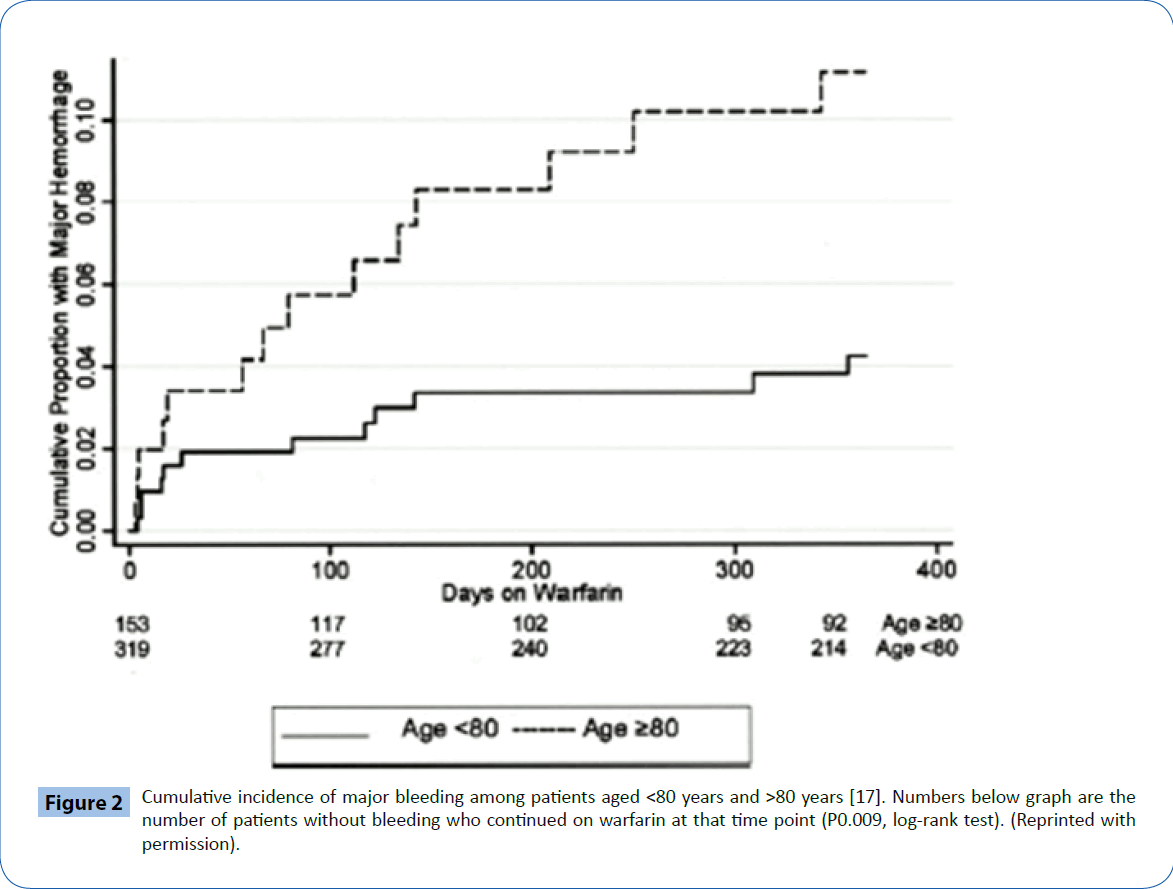
Figure 2: Cumulative incidence of major bleeding among patients aged <80 years and >80 years [17]. Numbers below graph are the number of patients without bleeding who continued on warfarin at that time point (P0.009, log-rank test). (Reprinted with permission).
Intracranial hemorrhage
Intracranial hemorrhage (ICH) is the most dreaded type of hemorrhage in any patient on OAC. Though the risk of ICH increases with age, the absolute risk of warfarin-associated ICH is relatively low at around 0.2% to 0.6% per year [18]. However, a post-hoc analysis of the ATRIA study by Fang et al. [19] showed that warfarin-associated ICH had a very high mortality rate (60% in the first 30 days).
McGrath et al. [20] evaluated 3,197 patients with AF and an acute stroke from the Registry of the Canadian Stroke Network to assess risk factors for ICH. The odds ratios for age (OR, 1.19; 95% CI, 1.06–1.34 per decade), and prior stroke or TIA (OR, 1.45; 95% CI, 1.12–1.87) were higher for ischemic stroke compared to ICH. An elevated INR at the time of admission was more strongly associated with ICH than ischemic stroke (INR>3.0 versus <1.4, OR, 0.07; 95% CI, 0.04–0.10). The Stroke Prevention in Atrial Fibrillation investigators (SPAF) [21] found that a systolic BP of >160 mm Hg and a diastolic BP of >90 mm Hg increases the risk of ICH in patients on anticoagulation (RR of 4). Head trauma in a patient on anticoagulation therapy can increase the likelihood of ICH (subdural hematoma). Despite this risk, a recent study [22] found that persons taking warfarin must fall around 295 times in one year for the risk of warfarin therapy to outweigh the potential benefit. Nevertheless, elderly patients with a supra-therapeutic INR (INR>3) and/or a history of previous stroke/TIA are at a very high risk for ICH. Falls in this population can result in serious consequences, including, but not limited to, ICH. The highest incidence of ICH is seen in elderly patients with evidence of cerebral amyloid deposition with microbleeds. Several studies have evaluated the use of MRI imaging of the brain and cerebrospinal fluid antibodies and total tau levels to identify patients at risk. However, further research is needed in this regard before any practical application of these tests can be adopted to assess patients at the highest risk for ICH [23].
Assessment of bleeding risk
Several bleeding risk scores have been developed to stratify risk of hemorrhage, including the HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol abuse), RIETE (Computerized Registry of Patients With Venous Thromboembolism), HEMORR2HAGES (Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Rebleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke), and ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation). Although HAS-BLED discriminates bleeding risk better than the rest, all of these scoring systems have a modest predictive ability for bleeding (c-statistic ≈ 0.6) [24].
The 2014 AHA/ACC/HRS guideline states that, although these scoring systems may be useful in determining bleeding risk, given that all these scoring systems have poor predictive capabilities and a low C-index, there is insufficient evidence to provide recommendations for their use in clinical practice. The HASBLED score has been validated in a prospective study where ‘real world’ patients with AF were followed for one year and the predictive capability compared to rates of major bleeding [25]. Thus, HAS-BLED has been recommended by the ESC and CCS as the scoring system of choice, as it is much simpler to use than the other systems. A patient with a HAS-BLED score >3 is considered to be at a high risk for bleeding and requires regular monitoring and caution while initiating OAC. A high HAS-BLED score in itself does not preclude an elderly patient from OAC therapy; rather, efforts should be made to control reversible risk factors such as uncontrolled BP, use of non-steroidal anti-inflammatory drugs, labile INRs etc.
Improving efficacy of warfarin therapy in the geriatric population
Older patients are more sensitive to small changes in warfarin dosing, and therefore should be started on lower initial doses as recommended in the American College of Chest Physicians (ACCP) guidelines. Algorithm-consistent dosing is a strong predictor of TTR, as shown in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial (10% increase in algorithm-consistent dosing predicted a 6.12% increase in TTR) [26]. Patient education, identifying patient-level barriers to adherence to warfarin therapy, and prompt follow-up when INR values are out of range are other ways to improve the efficacy of warfarin therapy.
Use of antiplatelet therapy for prevention of stroke in elderly AF patients
The Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE)-W, and ACTIVE-A trials [27,28] showed that warfarin was superior to the combination of clopidogrel and ASA for preventing strokes. The ACTIVE A (Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation) trial [29] enrolled 7554 AF patients (mean age 71 years) who were considered unsuitable for warfarin therapy and assigned patients to ASA+clopidogrel versus ASA. ASA+clopidogrel decreased the risk of stroke (RR 0.72, P<0.001), and increased the risk of major hemorrhage (RR 1.57, P<0.001) compared to ASA alone. The reasons for patients being deemed unfit for warfarin therapy included physician judgment (50.4%), patient’s preference not to take warfarin (26%), and specific risk for bleeding (23%).
The 2012 European Society of Cardiology (ESC) focused update [30] recommended that combination antiplatelet therapy should be limited only to patients who refuse OAC. The Canadian Cardiovascular Society (CCS) guidelines recommend using dabigatran in those considered unsuitable for warfarin (Table 2). Except for the Stroke Prevention in Atrial Fibrillation-1 (SPAF) study, no other studies have demonstrated any significant reduction in stroke risk with ASA. Moreover, in the SPAF-1 study, ASA did not decrease the risk of stroke in patients >75 years of age. In addition, there was an increased rate of adverse events, including bleeding, with ASA in patients aged 80-89 years in the Warfarin versus Aspirin for Stroke Prevention in Octogenarians (WASPO) trial [31]. Accordingly, the 2014 ACCF/AHA/HRS guidelines do not offer any specific recommendations regarding the use of antiplatelet therapy for stroke prevention in AF.
Triple anti-thrombotic therapy and bleeding risk
The prevalence of coronary artery disease (CAD) rises with age, and managing elderly AF patients with CAD can be challenging, especially if they have to undergo percutaneous coronary intervention (PCI). Sorenson et al. [32] reported bleeding rates of 5.1% for aspirin plus warfarin, 12.3% for clopidogrel plus warfarin, and 12.0% for triple therapy in a cohort of 40,812 patients from a National Danish patient registry. Ruiz-Nodar et al. [33] studied 590 patients with AF with CHA2DS2-VASc scores of >1 undergoing PCI. Of the study cohort, 420 (71%) patients had a HAS-BLED score >3. In these patients, OAC use at discharge (as part of combination therapy) had a significantly lower mortality rate but a much higher bleeding rate. The risks for stroke, major bleeding, and stent thrombosis have to be weighed before making a decision regarding combination therapy. The AHA/ ACC/HRS guideline recommends the use of clopidogrel with OACs but without aspirin following coronary revascularization. Various societies have proposed guidelines regarding the use of triple therapy in AF patients, the key points of which have been summarized in Table 3.
| ACCF/AHA/HRS guidelines |
ESC guidelines |
ACCP guidelines |
CCS guidelines |
1. Stable CAD:
No specific recommendations. |
1. Stable CAD:
In patients with stable CAD and no acute events for >1 year, warfarin monotherapy is recommended (Class IIB.If elective PCI is carried out, a BMS should preferably be used over DES unless a significant benefit is expected with a DES (Class IIA). |
1. Stable CAD:
In AF patients with stable CAD, warfarin therapy alone is recommended over combination therapy. |
1.Stable CAD:
In AF patients with -
CHADS2 score =0, ASA may be used.
CHADS2 score >1, OACmonotherapy. |
2. Following PCI:
a)BMS:
Warfarin + Clopidogrel for at least 1 month. b) DES:
Warfarin + Clopidogrel for 3 months after a sirolimus eluting stent, and 6 months after a paclitaxel eluting stent. Warfarin + Clopidogrel may be continued for longer duration (12 months) in selected patients, followed by VKA monotherapy. |
2. Following PCI:
a) BMS-
Triple therapy1 may be used for a minimum of one month.
b)DES-
Triple therapy for 3 months for a sirolimus eluting stent, and 6 months for a paclitaxel eluting stent should be considered.
Following this dual therapy with warfarin+ Clopidogrel or ASA (75-100 mg daily + PPI/ H2 blockers) for up to 1 year may be considered after assessing individual bleeding risks. |
2.Following PCI:
AF patients with a CHADS2 score of >2
a) BMS:
Triple therapy for 1 month.
b) DES:
Triple therapy for 3-6 months.
After this initial period dual therapy (warfarin+ ASA/ Clopidogrel) may be used for upto 12 months. |
2. Following PCI:
BMS/ DES-
In AF patients with-
CHADS2<1, ASA + Clopidogrel.
CHADS2 score >2, triple therapy may be considered. |
| 3. Following PCI, warfarin + ASA and/ or Clopidogrel may be given keeping in mind that these strategies are associated with an increased risk for bleeding. |
3. Following an ACS:
Triple therapy for 3-6 months (longer in a patient with low bleeding risk), followed by long term dual therapy with warfarin + Clopidogrel or ASA + gastric protection (Class IIA). |
3. For AF patients with a CHADS2 score < 2, dual therapy may be used for the first 12 months after placement of either a BMS or a DES. |
3. The CCS guidelines do not specify the duration of therapy after PCI, however they state that the decision should be based on assessment of the relative risks of stroke, stent thrombosis, and hemorrhage. |
| |
4. When warfarin + Clopidogrel or ASA is prescribed a carefully regulated INR range of 2.0-2.5 may be appropriate. |
|
4. In AF patients with CAD at a high risk for coronary events warfarin may be preferred over dabigatran. |
| AF: Atrial fibrillation; ACCF/ AHA/ HRS: American College of Cardiology/ American Heart Association/ Heart Rhythm Society; ACCP: American College of Chest Physicians; ASA: Aspirin; BMS: Bare metal stent; CAD: Coronary artery disease; CCS: Canadian Cardiovascular Society; CHADS2: Congestive heart failure, Hypertension, Age > 75, Diabetes Mellitus, Stroke/ TIA; DES: Drug eluting stent; ESC: European Society of Cardiology; OAC: Oral Anticoagulation; PCI: Percutaneous coronary intervention; PPI: Proton pump inhibitor; VKA: Vitamin K Antagonists; Triple therapy – VKA + ASA+ Clopidogrel. |
Table 3: The ACCF/AHA/HRS [5], ACCP [7], ESC [8], and CCS [6] guidelines for antithrombotic therapy in AF patients with CAD.
Newer Anticoagulants
Dabigatran
Dabigatran is a direct thrombin inhibitor approved in 2010 by the U. S. Food and Drug Administration (FDA). It undergoes 80% renal elimination and must be used cautiously in renal impairment. The RE-LY trial [34] randomized 18,113 patients to dabigatran 150 mg (D150) twice daily, dabigatran 110 mg (D110) twice daily, or open-label warfarin. The mean age, CHADS2 score, and TTR of the patients were 70 years, 2.1, and 64% respectively (Table 4). Eightytwo percent of patients were aged >65 years and 40% were aged >75 years. The rates of stroke/SE were 1.53%/year for D110, 1.11% per year for D150, and 1.69% per year for warfarin [35]. Both doses of dabigatran were non-inferior to warfarin, and the 150 mg dose of dabigatran was superior to warfarin (RR, 0.66; 95% [CI], 0.53 to 0.82; P<0.001). Both doses of dabigatran were associated with a lower risk for ICH when compared to warfarin, across all age groups. However, in patients >75 years of age, both doses of dabigatran were associated with a higher risk for extracranial bleeding, particularly gastrointestinal (GI) bleeding (RR 1.39 (P=0.02) and 1.79 (P=0.06) for D110 and D150 dabigatran respectively) [36].
| Patient and drug characteristics |
Dabigatran (RE-LY trial) [34] |
Rivaroxaban (ROCKET-AF trial) [41] |
Apixaban (ARISTOTLE trial) [44] |
Edoxaban
(ENGAGE-AF TIMI 48) [46] |
| Total patients enrolled |
18,111 |
14,264 |
18,201 |
21,105 |
| Mean age (years) |
71.5 ±8.7 |
73 (65-78) |
70 (63-76) |
72 (64-78) |
| Percentage of patients > 75 years of age (%) |
40 |
38 |
31 |
40 |
| Average body weight (kg) |
82.5 ± 19 |
BMI (Body Mass Index):
: 28.3±4 |
82 ± 14 |
> 60 kg in 90% of the patients |
Mean
CHADS2 score |
2.1 |
3.5 |
2.1 |
2.8 |
| Dose |
150 mg bid |
20 mg once daily |
5 mg bid |
30-60 mg daily |
| Dose adjusted for renal impairment |
75 mg bid for CrCl 15-30 ml/min (US only) |
15 mg once daily |
2.5 mg bid |
Dose halved for CrCl 30-50 ml/ min |
| Dose under special circumstances |
110 mg bid (ESC and CCS) in patients > 80 years of age, HAS-BLED≥3, use of interacting drugs, renal impairment. |
15 mg once daily in patients with HAS-BLED score ≥3, and renal impairment. |
2.5-mg in patients with two or more of the following criteria: ≥80 years, body weight ≤60 kg, or renal impairment |
Dose halved for CrCl of 30 - 50 ml/min, a body weight of ≤ 60 kg or concomitant use of verapamil,quinidine or dronedarone |
| Half-life (hours) |
12-17 (CrCl 50-80 ml/min)
18-24 (CrCl 30-49 ml/min)
>24 (CrCl<30 ml/min) |
5-13 |
9-14 |
6-10 |
| Excretion |
Renal (80%) |
Renal (1/3), liver (2/3) |
Renal (25%), fecal (75%) |
Renal (35%),fecal (65%) |
| Target |
Thrombin |
Factor Xa |
Factor Xa |
Factor Xa |
| CHADS2: Congestive heart failure, Hypertension, Age > 75, Diabetes Mellitus, Stroke/ TIA; CrCl: Creatinine Clearance; ESC: European Society of Cardiology; CCS: Canadian Cardiovascular Society; HAS-BLED: Hypertension, Abnormal renal or liver function , Stroke , history of Bleeding , Labile INRs, Elderly, Drug or alcohol abuse; OAC: Oral Anticoagulation therapy; ACCF/ AHA/ HRS: American College of Cardiology/ American Heart Association/ Heart Rhythm Society; ACCP: American College of Chest Physicians; FDA: Food and Drug Administration. |
Table 4: Newer anticoagulants.
In particular, Graham et al. [37] studied elderly patients (>65 years) on oral anticoagulants for AF and found a decreased risk for ischemic stroke (HR: 0.80; 95% CI: 0.67-0.96) and ICH (HR: 0.34; 94% CI: 0.26-0.46) in patients on dabigatran as compared to a propensitymatched cohort of patients on warfarin. The investigators also found an increased risk for major GI bleeding (HR: 1.28; 95% CI: 1.14- 1.44) with dabigatran as compared to warfarin. However, the study did not take into account the concomitant use of aspirin and over the counter non-steroidal anti-inflammatory drugs. Also, the time in therapeutic range (TTR) with warfarin could not be ascertained, although the TTR probably reflected that seen in general practice.
A higher incidence of dyspepsia was noted in patients on dabigatran as compared to warfarin in the RE-LY trial. Dabigatran requires a low pH for good absorption, and in order to achieve this, the capsules contain tartaric acid cores coated on the outside with dabigatran, which has been postulated as the cause for increased dyspepsia and possibly GI bleeding.
There have been concerns regarding the increased risk of myocardial infarction (MI) associated with the use of dabigatran. A meta-analysis [38] evaluated this risk and found that dabigatran use was associated with a statistically significant higher risk of MI compared to warfarin. With the 150 mg BID dosage the odds ratio for MI when compared with warfarin was 1.43 (95% CI 1.08 to 1.89; P= 0.152), and with the 110 mg BID dosage it was 1.33 (95% CI 0.99 to 1.77; P=0.760).
Dosing with older age and impaired renal function
The 150 mg dose of dabigatran was approved by the FDA, [39] but not the 110 mg dose. Prior to arriving at this decision, the FDA analyzed high risk groups in the RE-LY study (elderly, patients with renal insufficiency) and found that among patients >75 years of age (40% of the total cohort), the rate of stroke/SE was lower with D150 (1.4%/year) than with D110 (1.9%/year), though the rate of major bleeding was higher with the D150 dose (5.1 vs. 4.4 %/patient/year). Based on this analysis, the D150 dose was found to be more efficacious across all groups of patients. The FDA was concerned that the D110 dose might be preferred more often by physicians concerned more about prevention of bleeding in elderly patients than prevention of TE events. Reilly et al. [40] studied the effects of dabigatran plasma concentration and age on the incidence of ischemic stroke and major bleeds. They found that in patients with major bleeding events, the median trough and post-dose concentrations were 55% and 36% higher, respectively, than in those without bleeding events. Renal function (CrCl) and age were found to be the two most important determinants of the plasma concentration of dabigatran, with a 68% higher trough concentration in patients >75 years of age as compared to those <65 years of age. In addition, low body weight and female sex were associated with higher plasma concentrations of dabigatran. This substudy showed that across the 10th to 90th percentiles of plasma trough concentrations, the risk of major bleeding ranged from 2-7%, with increasing age as an important contributing factor. The risk for ischemic stroke across the same concentrations ranged from ~1.5% in patients <75 years of age to ~2.1% for patients >80. In frail elderly patients, especially in those with impaired renal function, a lower daily dose might be more ideal, given the decreased risk of major bleeds without loss of efficacy in terms of preventing embolic stroke.
Prior to initiating therapy with dabigatran, the risk-benefit ratio should be assessed for all patients, especially the elderly. The mean age and weight of patients enrolled in the RE-LY trial was 70 years and 82 kg respectively, and in frail elderly patients, especially those >80 years of age with some degree of renal insufficiency, the D150 dose may pose an increased risk of bleeding. This risk may also be exacerbated by concomitant medications like verapamil and amiodarone (P-glyprotein inhibitors). As described above, a subset of geriatric patients may be at a higher bleeding risk with both the D150 dose as well as warfarin therapy. In such patients, other dosing options might warrant further study. The latest guidelines by the ACCF/AHA/HRS, ACCP, ESC, and CCS in regards to the newer anticoagulants are summarized in Table 4.
Rivaroxaban
Rivaroxaban is a direct factor Xa inhibitor predominantly metabolized by the liver, with one-third of the drug undergoing renal elimination. The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) [41] randomized 14,264 patients with AF and a CHADS2 score of ≥ 2 to rivaroxaban (20 mg daily or 15 mg daily in patients with a CrCl of 30-49 ml per minute) or warfarin. ROCKET-AF had a significantly sicker population than RE-LY and the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) (ARISTOLE) trial [42]. The mean age of the patients was 73 years, with a mean CHADS2 score of 3.5. It should be noted that the TTR for the ROCKET-AF trial overall was lower as compared to other randomized controlled trials at 55%. In the primary analysis, the rivaroxaban group had a lower rate of stroke/SE (1.7% per year) than those in the warfarin group (2.2% per year) (HR 0.79; 95% CI; 0.66-0.96; P<0.001 for non-inferiority). Rates of major bleeding were similar for rivaroxaban and warfarin (14.9% vs. 14.5%, HR: 1.03, CI 0.96-1.11; P=0.44), although major bleeding from a gastrointestinal site was higher in the rivaroxaban group (3.2 vs 2.2%, P<0.001). Rates of ICH were lower in the rivaroxaban group than in the warfarin group (0.5% vs. 0.7% per year, P=0.02). The ROCKET-AF trial had a higher rate of early treatment discontinuation (around 14.3% in the first year), which was higher compared to the other major trials (around 10-11%). Halperin et al. [43] studied 6,229 elderly patients (>75 years of age) as a part of a pre-specified secondary analysis of the ROCKET-AF trial and found no difference in the primary efficacy outcome (stroke/ SE) between warfarin therapy and rivaroxaban. Similarly, there were no differences in major bleeding events between the warfarin and rivaroxaban groups. However, the incidence of GI bleeding was higher in patients treated with rivaroxaban compared to warfarin.
Apixaban
Apixaban is a direct factor Xa inhibitor recently approved by the FDA for stroke prevention in AF. The ARISTOTLE trial [44] randomized 18,201 patients with AF and one additional risk factor for stroke to apixaban or dose-adjusted warfarin. The median age of the population was 70 years with 31% of the population >75 years of age. The mean CHADS2 score was 2.1, and the mean TTR of the warfarin cohort was 66%. The primary outcome of stroke/SE occurred in 1.27% patients/year in the apixaban group versus 1.60% per year in the warfarin group (HR for apixaban 0.79; 95% CI, 0.66 to 0.95; P<0.001 for non-inferiority and P = 0.01 for superiority). The rate of ICH was 0.33% per year in the apixaban group and 0.80% per year in the warfarin group (HR, 0.42; P<0.001). The clinical benefit of apixaban over warfarin was consistent across all patients irrespective of their CHADS2, CHA2DS2-VASc, and HAS-BLED scores. In addition, apixaban decreased all-cause mortality when compared to warfarin.
The Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial [45] showed greater efficacy for apixaban in preventing stroke/SE events and similar rates of bleeding compared to ASA, making this a good option in elderly patients considered unsuitable for warfarin therapy.
Dosing with older age and impaired renal function
Apixaban was given 5 mg twice daily in the ARISTOTLE study, although 2.5 mg twice daily was used in a subset of patients with two or more of the following criteria: ≥80 years, body weight ≤ 60 kg, or a serum creatinine level of ≥ 1.5 mg/dL. The superiority of apixaban relative to warfarin for preventing stroke was consistent, irrespective of the degree of renal impairment [44].
Edoxaban
The Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 [46] (ENGAGE-AF TIMI 48) randomized 21,105 patients to warfarin, high dose or low dose edoxaban. Patients received low dose edoxaban if any one of the following was present: estimated CrCl of 30- 50 ml/ minute, weight of ≤ 60 kg, or the concomitant use of verapamil, dronedarone or quinidine. Both doses of edoxaban were found to be non-inferior to warfarin for the prevention of stroke and systemic embolism (rate of stroke or systemic embolic event for warfarin vs high dose edoxaban was 1.50% vs 1.18% per year, HR: 0.79; 97.5% CI, 0.63 to 0.99; P<0.001 for non-inferiority). The rates of major bleeding, including ICH, were lower for edoxaban versus warfarin, except that the rate of major GI bleeding was higher with high dose edoxaban compared to warfarin. The mean age of the study group was 72 years, with around 40% of the population ≥ 75 years of age. However, there was a higher risk of ischemic stroke with edoxaban in patients with a CrCl > 95 ml/ min compared with warfarin leading to the current labeling that edoxaban should not be used in this subgroup of patients.
Advantages of newer anticoagulants over warfarin
Advantages of the newer OACs include predictable pharmacodynamic effects, good efficacy and safety profiles without the need for anticoagulation monitoring, rapid onset of action (2-4 hours vs ~ 72 hours for warfarin), and shorter half-lives that make the use of these agents in the peri-procedural period much easier (Table 5). The newer anticoagulants have also been consistently found to reduce the risk of ICH as compared to warfarin irrespective of age.
| Warfarin [47] |
Newer OACsNewer OACs |
1. Warfarin therapy should be stopped approximately five days prior to surgery
2. Warfarin therapy may be started 12 to 24 hours after surgery, when there is adequate hemostasis.
3. Bridging therapy with low molecular weight (LMW) heparin is recommended for patients with a mechanical heart valve, atrial fibrillation, or VTE at a high risk for thromboembolism. |
1. The optimal timing prior to surgery for stopping the newer OACs depends on the patient’s renal function, and the risk of bleeding associated with the procedure. Optimal timing prior to surgery to stop newer OACs[48]
| CrCl |
> 80 |
50-79 |
30-49 |
15-29 |
| Risk of bleeding |
L |
H |
L |
H |
L |
H |
L |
H |
| Dabigatran (duration in hours) |
>24 |
>48 |
>36 |
>72 |
>48 |
>96 |
CI |
CI |
| Rivaroxaban (duration in hours) |
>24 |
>48 |
>24 |
>48 |
>24 |
>48 |
>36 |
>48 |
| Apixaban (duration in hours) |
>24 |
>48 |
>24 |
>48 |
>24 |
>48 |
>36 |
>48 |
Newer OACs are contraindicated in patients with CrCl< 15 ml/min [50]. |
| CI: Contraindicated; CrCl: Creatinine Clearance; L: Low; H: High; OAC: Oral Anticoagulation therapy; VTE: Venous Thromboembolism |
Table 5: Peri-operative/pre-procedural protocol in patients on OAC therapy.
Risks with newer anticoagulants
Warfarin has been in use as an anticoagulant for several decades now. Though there are problems associated with warfarin use, these are well known, and they have been studied extensively both in clinical trials and in real world practice. Many patients started on OACs require these medications for an extended period of time, often lifelong. We do not have long-term data for the newer anticoagulants. Only one-third of the patients enrolled in the newer anticoagulant clinical trials were >75 years of age. Moreover, ‘frail’ elderly patients and patients with significant renal disease were not included in these trials. There is a risk for major bleeding even with the newer anticoagulants, especially in the frail elderly. In a recent study [49] analyzing the risk of major bleeding with dabigatran in real world practice in New Zealand over a two month period, the predictors of bleeding were incorrect prescription, impaired renal function, and patient age. Currently, the newer anticoagulants do not have FDA-approved antidotes to reverse bleeding. However, a fully humanized antibody fragment called idarucizumab was recently shown to reverse the anticoagulant effects of dabigatran in healthy volunteers, and further large scale studies are underway [51,52]. The management of bleeding with the newer anticoagulants is mainly supportive, as described in Table 6. High cost may also be a limiting factor for the newer anticoagulants, although D150 was found to be cost effective in patients with high CHADS2 scores and an inability to maintain stable INRs [53].
| Management of major bleeding in a patient on oral anticoagulation [50,51] |
| Supportive therapy in the form of fluid resuscitation, blood transfusion, investigation for the source of bleeding, mechanical compression and where appropriate surgical hemostasis should be undertaken. |
| Fresh frozen plasma (FFP) or prothrombin complex concentrates (PCC) can be used to reverse the effect of warfarin in case of a major bleed. Vitamin K can be administered concomitantly, though it takes a longer time to reverse the anticoagulant effect. |
| There are no specific antidotes available in the market for the newer anticoagulants although a reversal agent for dabigatran is being studied currently. However these agents have shorter half-lives, and their anticoagulant action does not last long after discontinuation of the drug. |
| In cases of life threatening bleeding in a patient on Dabigatran, prothrombin complex concentrates (PCC) or recombinant activated factor VIIa may be considered though the clinical utility of these agents has not been established. |
| Adequate diuresis should be maintained as Dabigatran is predominantly renally excreted. |
| In cases of severe bleeding, or overdose of Dabigatran, when rapid reversal is required hemodialysis can be carried out to facilitate plasma clearance of Dabigatran. |
Table 6: Management of bleeding events in patients on oral anticoagulation.
Although these drugs are recommended in patients with unstable and difficult to maintain INRs, it is important to determine the reason for the unstable INRs. If the problem is poor compliance with medications, and the patient cannot be trusted to take the drug regularly, the newer OACs may not be a better choice in these patients, as they have a much shorter half-life and missing a dose or two can result in an increased thromboembolic risk.
Further Research
While many studies have been done comparing newer OACs to warfarin, there are no head-to-head comparisons of any of the newer OACs with each other. Patients with renal impairment have a very high risk for both stroke and major bleeding and the incidence of renal dysfunction is higher in the elderly than in the younger population. Patients with advanced renal failure and/ or on dialysis were not included in any of the major newer OAC trials and as of now warfarin is the only option available for these patients. Also, as noted above, triple antithrombotic therapy in elderly patients with AF and acute coronary syndrome is problematic, as it confers a high risk of major bleeding. With the advent of newer antiplatelet agents such as ticagrelor and prasugrel, further studies with these agents in patients with AF and ACS requiring triple therapy are warranted.
Conclusions
Elderly patients with AF are at a particularly high risk for TE complications. With a large fraction of such patients not receiving anticoagulation, the recent Introduction of newer anticoagulants may improve rates of treatment by providing different options for management. For an elderly patient who has been on warfarin for a long time with good TTRs, no major complications, and ensured compliance with regular testing, it may be appropriate to continue with warfarin based on the patient’s preference. On the other hand, the newer OACs have convenient dosing, no need for blood testing for efficacy, and may be particularly attractive in patients at high risk for stroke in whom the TTR is sub-optimal [54].
Patients place a greater emphasis on stroke prevention and a lower value on avoiding bleeding than do physicians who treat AF [55]. It is very important to take into account patient preference before making a decision regarding starting or deferring anticoagulant therapy for a patient. The risk of stroke, risk of major bleeding, and the need for regular INR monitoring if on warfarin should be explained clearly. The addition of the newer OACs to the therapeutic options available for stroke prevention in AF has provided patients and clinicians with an attractive alternative choice of therapy which does not require regular monitoring, has a similar or better safety profile with respect to major bleeding (especially ICH), and can be conveniently dosed. At the same time as these newer OACs have only been around for a short time, their long term performance data is yet to be fully evaluated. It has been shown in several studies [56] that AF patients with similar risk profiles choose different stroke prevention strategies, and it is only appropriate that patient preferences are included when making this important decision.
7037
References
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, et al. (2008) Heart disease and stroke statistics-2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25-146.
- Go AS, HylekEM, Phillips KA, Chang Y, Henault LE, et al. (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285: 2370-2375.
- Wolf PA, Abbott RD, Kannel WB (1987) Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 147: 1561-1564.
- Jørgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS (1996) Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 27: 1765-1769.
- January CT, Wann L, Alpert JS (2014) 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 130: 2071-2104.
- Skanes AC, J S Healey (2012) Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol 28: 125-136.
- GuyattGH, AklEA, Crowther M (2012) Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: 48S-52S.
- CammAJ, Kirchhof P, Lip GY (2010) Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369-2429.
- Mant J, Hobbs FD, Fletcher K (2007) Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 370: 493–503.
- Fang MC, Go AS, HylekEM, Chang Y, Henault LE, et al. (2006) Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am GeriatrSoc 54: 1231-1236.
- Schulman S, Beyth RJ, Kearon C, Levine MN (2008) Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 133: 257S-298S.
- Singer DE, Chang Y (2009) Should Patient Characteristics Influence Target Anticoagulation Intensity for Stroke Prevention in Nonvalvular Atrial Fibrillation?:The ATRIA Study. Circulation: Cardiovascular Quality and Outcomes 2: 297-304.
- Cafolla A, Campanelli M, Baldacci E, Potasso L, Bochicchio R, et al. (2012) Oral anticoagulant therapy in Italian patients 80 yr of age or older with atrial fibrillation: a pilot study of low vs. standard PT/INR targets. Eur J Haematol 89: 81-86.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, et al. (2009) Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 124: 37-41.
- Poli D, Antonucci E (2011) Bleeding risk in very old patients on vitamin K antagonist treatment: results of a prospective collaborative study on elderly patients followed by Italian Centres for Anticoagulation. Circulation 124: 824-829.
- HylekEM, Evans-Molina C, Shea C, Henault LE, Regan S (2007) Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 115: 2689-2696.
- Gomes T, Mamdani M (2012) Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ 85: E121-E127.
- Hart RG, Tonarelli SB, Pearce LA (2005) Avoiding central nervous system bleeding during antithrombotic therapy: recent data and ideas. Stroke 36: 1588-1593.
- Fang MC, Go AS, Chang Y, BorowskyLH, PomernackiNK, et al. (2012) Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 43: 1795-1799.
- McGrath ER, Kapral MK, Fang J, EikelboomJW, ó Conghaile A, et al. (2012) Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 43: 2048-2054.
- (1996) Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Arch Intern Med 156: 409-416.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A (1999) Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 159: 677-685.
- Lee SH, Ryu WS, RohJK (2009) Cerebralmicrobleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology 72: 171-176.
- Lip GY, Banerjee A, Lagrenade I, Lane DA, Taillandier S, et al. (2012) Assessing the risk of bleeding in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation project. CircArrhythmElectrophysiol 5: 941-948.
- OlesenJB, Lip GY, Hansen PR, Lindhardsen J, Ahlehoff O, et al. (2011) Bleeding risk in 'real world' patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J ThrombHaemost 9: 1460-1467.
- Van Spall HGC, Wallentin L, Yusuf S(2012) Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries: an analysis of patients receiving warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 126:2309 –2316.
- ACTIVE Writing Group of the ACTIVE Investigators, Connolly S, Pogue J, Hart R, Pfeffer M, et al. (2006) Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 367: 1903-1912.
- ACTIVE Investigators, Connolly SJ, Pogue J, Hart RG, HohnloserSH, et al. (2009) Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 360: 2066-2078.
- ACTIVE Investigators, Connolly SJ, Pogue J, Hart RG, HohnloserSH, et al. (2009) Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 360: 2066-2078.
- Rash A, Downes T, Portner R, Yeo WW, Morgan N, et al. (2007) A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO). Age Ageing 36: 151-156.
- CammAJ, Lip GY(2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association.Eur Heart J 33: 2719-2747.
- Sørensen R, Hansen ML, AbildstromSZ, Hvelplund A, Andersson C, et al. (2009) Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet 374: 1967-1974.
- Ruiz-NodarJM, Marin F (2012) Should we recommend oral anticoagulation therapy in patients with atrial fibrillation undergoing coronary artery stenting with a high HAS-BLED bleeding risk score?CircCardiovascInterv 5: 459-466.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139-1151.
- EikelboomJW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, et al. (2011) Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 123: 2363-2372.
- Graham DJ, Reichman ME, Wernecke M, Zhang R, SouthworthMR, et al. (2015) Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 131: 157-164.
- Douxfils J, Buckinx F (2014)DabigatranEtexilate and Risk of Myocardial Infarction, Other Cardiovascular Events, Major Bleeding, and All-Cause Mortality: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Journal of the American Heart Association 3: e000515.
- Beasley BN, Unger EF, Temple R (2011) Anticoagulant options--why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med 364: 1788-1790.
- Reilly PA, Lehr T (2014)The Effect of Dabigatran Plasma Concentrations and Patient Characteristics on the Frequency of Ischemic Stroke and Major Bleeding in Atrial Fibrillation Patients: The RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). Journal of the American College of Cardiology 63: 321-328.
- PatelMR, Mahaffey KW, Garg J (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883-891.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, HylekEM, et al. (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981-992.
- Halperin J, Hankey G (2014) Efficacy of rivaroxaban to prevent stroke in atrial fibriallation maintained in elderly. Circulation.
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, et al. (2011) Apixaban in patients with atrial fibrillation. N Engl J Med 364: 806-817.
- Lopes RD, Al-Khatib SM, Wallentin L, Yang H, Ansell J, et al. (2012) Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet 380: 1749-1758.
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, et al. (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369: 2093-2104.
- Douketis JD, Spyropoulos AC (2012) Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines.Chest 141: e326S-e350S.
- Healey JS, Eikelboom J (2012) Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 126: 343-348.
- Harper P, Young L, Merriman E (2012) Bleeding risk with dabigatran in the frail elderly. N Engl J Med 366: 864-866.
- Heidbuchel H, Verhamme P, Alings M, Antz M, HackeW, et al. (2013) EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 34: 2094–2106.
- Van Ryn J, Litzenburger T, Waterman A (2011)Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am CollCardiol57: E1130.
- Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, et al. (2013) A specific antidote for dabigatran: functional and structural characterization. Blood 121: 3554-3562.
- Shah SV, Gage BF (2011) Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 123: 2562-2570.
- Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, et al. (2011) Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 154: 1-11.
- DevereauxPJ, Anderson DR, Gardner MJ, Putnam W, FlowerdewGJ, et al. (2001) Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 323: 1218-1222.
- Man-Son-Hing M, Laupacis A, O'Connor A, Wells G, Lemelin J, et al. (1996) Warfarin for atrial fibrillation. The patient's perspective. Arch Intern Med 156: 1841-1848.








