Keywords
Sporadic Inclusion Body Myositis (sIBM); Myositis; Muscle biopsy; Rimmed vacuoles; Cytosolic 5’- nucleotidase 1A
Abbreviations
sIBM: Sporadic Inclusion Body Myositis; cN1A: Cytosolic 5'- nucleotidase 1A; CK: Creatine Kinase; HCV: Hepatitis C Virus; HTLV-I: Human T-cell Leukemia Virus Type I; EF: Ejection Fraction; ENMC: European Neuromuscular Center; PM: Polymyositis; DM: Dermatomyositis; PBS: Phosphate-Buffered Saline; DAB: Diaminobenzidine; H&E: Hematoxylin and Eosin; mGT: Modified Gomori Trichrome; NADH-TR: Nicotinamide Adenine Dinucleotide-Tetrazolium Reductase; MHC-I: Major Histocompatibility I; IVIg: Intravenous Immunoglobulin; p62/ SQSTM1: p62/Sequestosome 1
Background
Sporadic Inclusion Body Myositis (sIBM) is a progressive muscular disorder developing in the elderly [1]. In Western countries, sIBM is the most common myositis in persons older than 50 years. However, in Japan, the number of patients with sIBM has been reported to be increasing recently [2]. Autoimmune systems and degenerative mechanisms are thought to play an essential part in the development of sIBM, but its pathogenesis remains poorly understood [1-3]. Effective treatments for sIBM have yet to be established. Several sets of criteria have been proposed to diagnose sIBM, emphasizing clinical characteristics and pathological findings [1,4-6]. However, highly sensitive and specific pathological clues suggesting a diagnosis of sIBM have yet to be established. Recently, anti-cytosolic 5'-nucleotidase 1A (cN1A) antibodies were reported to be present specifically in sIBM in the United States [3]. cN1A is an enzyme catalyzing the conversion of adenosine monophosphate (AMP) into adenosine and phosphate. It has been reported to be abundant in skeletal muscle [7]. Anti-cN1A antibodies are expected to play an important role in the diagnosis of sIBM, but few pathological studies have evaluated anti-cN1A autoimmunity. We therefore studied Japanese patients with sIBM to evaluate the clinical course and to assess the usefulness of anti-cN1A antibodies as a diagnostic tool for sIBM.
Patients and Methods
Patients
Among all patients in whom muscle biopsies were performed in our institution from January 1991 through December 2014, we identified patients who had sIBM that met the European Neuromuscular Center (ENMC) criteria [4] for clinically defined IBM or probable IBM.
Patient characteristics, including sex, age at onset and at diagnosis, initial symptoms, presence of dysphagia, concurrent presence of heart disease or restrictive ventilatory impairment, drinking or smoking habits, and walking disability 5 years after disease onset were obtained by retrospectively reviewing the patients’ medical records. Walking status was classified into three groups: No handicap; use of a cane or rollator; or wheelchair bound. Laboratory data at diagnosis included serum creatine kinase (CK) levels, hepatitis C antibodies, human T-cell leukemia virus type I (HTLV-I) antibodies, and electromyography. Treatment methods and effectiveness were also obtained from the medical records.
Quantitative values are expressed as means ± SD (standard deviation). Categorical variables were analyzed by Fisher’s exact test. This study was approved by the Ethical Review Committee of Nara Medical University.
Muscle biopsy
Muscle biopsy was performed in all patients with sIBM. Biopsy specimens were frozen in liquid nitrogen-cooled isopentane for histochemical and immunohistochemical analyses. Frozen transverse serial sections 10 μm in thickness were stained with a battery of histochemical methods, including hematoxylin and eosin (H&E), modified Gomori trichrome (mGT), and nicotinamide adenine dinucleotidetetrazolium reductase (NADH-TR) stains [8]. In addition, we performed indirect immunofluorescence staining on 7-μmthick cryosections of muscle. These sections were dried for 40 min and fixed in acetone at 4°C for 10 min. After washing with 0.01M phosphate-buffered saline (PBS), the sections were incubated at 37°C for 90 min with primary mouse monoclonal IgG antibodies against histocompatibility complex class I (MHC-I), CD4, CD8, CD68, C5b-9, p62/sequestosome 1 (p62/ SQSTM 1), and rabbit polyclonal anti-cN1A (1:200 dilution, Cat. No. ab75101; Abcam, Cambridge, MA). The sections were subsequently incubated at room temperature for 1 hour with a secondary antibody, biotinylated goat anti-mouse IgG (Vector Laboratories, Burlingame, CA). The reaction was visualized with 3,3’-diaminobenzidine (DAB) as the substrate, yielding a brown reaction product. For the anti-cN1A antibodies, the presence of at least one rimmed vacuole or one perinuclear region with anti-cN1A deposition was regarded as positive. As disease control, we also examined muscle specimens obtained from 10 patients with polymyositis (PM), 10 patients with dermatomyositis (DM), and 10 morphologically normal controls. PM and DM were diagnosed in accordance with the ENMC criteria [9].
Results
Clinical findings
Among 282 patients with inflammatory myopathy, 35 (12%) were given a diagnosis of sIBM. Twenty-four of these patients had clinically defined IBM and 11 had probable IBM according to the ENMC criteria. The clinical features are summarized in Table 1. The median age at disease onset was 67 ± 7 (55-81) years, and that at diagnosis was 70 ± 7 years. The most common symptom at disease onset was lower limb weakness. At diagnosis, 11 (32%) patients had dysphagia, but could ingest food orally. Laboratory examinations showed that the mean serum CK level was 532 ± 662 IU/L. Four (11%) patients concurrently had heart disease (arrhythmias in 3 patients and dilated cardiomyopathy in 1). Twenty patients underwent spirometry, and 2 patients showed restrictive respiratory impairment (forced vital capacity <80%), but did not require artificial ventilation. Four (11%) patients concurrently had cancer of the stomach, colon, esophagus, or prostate gland, respectively. They had already been treated and were well controlled without any treatment at the diagnosis of sIBM. Among the 33 patients for whom the results of electromyography were available, 22 showed myogenic changes, 3 showed neurogenic changes, and 8 showed both types of changes. On magnetic resonance imaging (MRI) of limb muscles, all 26 patients examined showed typical findings of fat infiltration and atrophy of the quadriceps femoris muscles and/or high intensity in the flexor digitorum profundus muscles. Twelve (34%) patients received immunosuppressive agents, 8 (23%) received prednisolone, and 5 (14%) received intravenous immunoglobulins (IVIg). Clinical symptoms improved transiently in 5 patients given prednisone and in 4 given IVIg. One patient received both treatments, but symptoms did not improve. As of 5 years after onset, 12 (44%) patients can walk unaided.
| Variable |
Result |
| Gender, male, n(%) |
24/35 (69) |
| Age at onset, y, mean ± SD |
67 ± 7 |
| Age at diagnosis, y, mean ± SD |
70 ± 7 |
| First symptoms, n (%) |
- |
| Lower limb weakness |
28/35 (80) |
| Upper limb weakness |
6/35 (17) |
| Dropped head |
1/35 (3) |
| Dysphagia at diagnosis, n (%) |
11/35 (31) |
| Serum CK (IU/L), mean ± SD |
532 ± 662 |
| Social history, n (%) |
- |
| Smoking (Brinkman index >400) |
15/31 (48) |
| Drinking (ethanol intake >20g/day) |
9/31 (29) |
| Complications, n (%) |
- |
| HCV infection |
5/35 (14) |
| HTLV-I infection |
1/25 (4) |
| Heart disease |
4/35 (11) |
| Restrictive ventilatory impairment |
2/20 (10) |
| Electromyography, n (%) |
- |
| Myogenic changes |
22/34 (65) |
| Neurogenic changes |
3/34 (9) |
| Myogenic and neurogenic changes |
8/34 (24) |
| Pathological findings, n (%) |
- |
| Rimmed vacuoles |
32/35 (91) |
| Anti-cN1A positive |
31/35 (89) |
| Patients receiving treatment, n (%) |
- |
| Prednisolone |
8/35 (23) |
| Transient improvement |
5/8 (63) |
| Intravenous immunoglobulin |
5/35 (14) |
| Transient improvement |
4/5 (80) |
| Handicap for walking |
- |
| At 5 years after onset, n (%) |
- |
| None |
12/27 (44) |
| Canes or rollator |
14/27 (52) |
| Wheelchair |
1/27 (4) |
CK: Creatine Kinase; HCV: Hepatitis C Virus; HTLV-I:Human T-Cell Leukemia Virus Type I;cN1A:Cytosolic 5'-nucleotidase 1A.
Table 1: Clinical features of 35 Japanese patients with sporadic inclusion body myositis.
Muscle pathology
Muscle biopsy specimens were examined before treatment began in all 35 patients. On histochemical staining including H&E, mGT, and NADH-TR, all patients showed mild to severe variations in fiber size. Necrotic and regenerating fibers were seen in the endomysium. In some patients, fibrous tissue and fatty degeneration were remarkable. Rimmed vacuoles were seen in many fibers in 32 (91%) patients. Intermyofibrillar networks were disorganized in scattered fibers in most of the patients. On immunohistochemistry staining, MHC-I expression showed cytoplasmic positivity in endomysial fibers. Infiltration of CD8-positive T-cells around non-necrotic fibers or invasion into such fibers was seen in 31 (89%) patients. Anti-cN1A positivity was detected in perinuclear regions or vacuole rims (Figure 1) in 31 patients (89%).
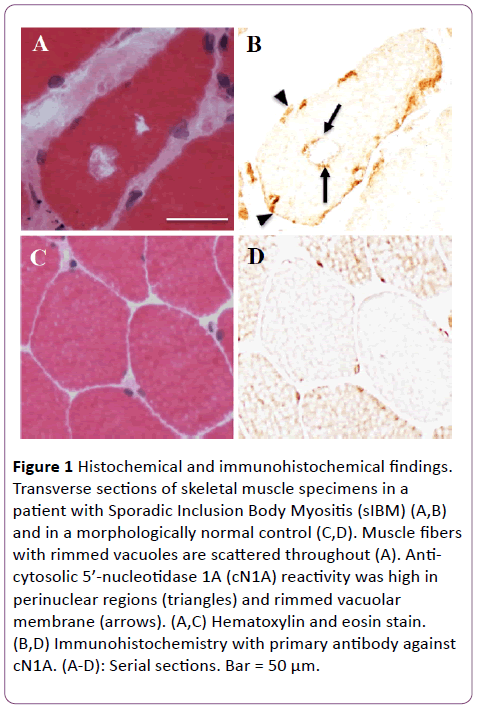
Figure 1: Histochemical and immunohistochemical findings. Transverse sections of skeletal muscle specimens in a patient with Sporadic Inclusion Body Myositis (sIBM) (A,B) and in a morphologically normal control (C,D). Muscle fibers with rimmed vacuoles are scattered throughout (A). Anticytosolic 5’-nucleotidase 1A (cN1A) reactivity was high in perinuclear regions (triangles) and rimmed vacuolar membrane (arrows). (A,C) Hematoxylin and eosin stain. (B,D) Immunohistochemistry with primary antibody against cN1A. (A-D): Serial sections. Bar = 50 μm.
We confirmed that p62/SQSTM1 was also expressed in these anti-cN1A positive vacuoles. Evaluation of serial sections with hematoxylin-eosin stain and anti-cN1A antibodies showed the accumulation of cN1A reactivity in these regions more clearly. On the other hand, muscle tissues of 3 patients with PM, 1 patient with DM, and none of the morphologically normal patients showed positivity for anti-cN1A antibodies (Table 2). Anti-cN1A positivity was significantly higher in patients with sIBM than in patients with PM (P<0.01) and patients with DM (P<0.01).
| ? |
Expressing region |
| ? |
Vacuolar rim |
Perinuclear region |
| sIBM, n=35 |
28 (80%) |
31 (89%) |
| PM, n=10 |
0 (0%) |
3 (30%) |
| DM, n=10 |
0 (0%) |
1 (10%) |
| morphologically normal, n=10 |
0 (0%) |
0 (0%) |
sIBM: sporadic inclusion body myositis, PM: polymyositis, DM: dermatomyositis
Table 2: Summary of pathological expression of anti-cN1A antibodies in sporadic inclusion body myositis, polymyositis, dermatomyositis, and morphologically normal patients.
Discussion
No previous study has surveyed both clinical data and detailed muscle pathological findings in a series of Japanese patients with sIBM. To our knowledge, our study is the first to evaluate the clinicopathological features of sIBM and demonstrate the usefulness of pathological anti-cN1A reactivity in Japanese patients.
The most important finding of our study is that anti-cN1A positivity in muscle tissue might lead to a diagnosis of sIBM with high sensitivity and specificity. Pathologically, sIBM is characterized by necrotic and regenerating fibers, cellular infiltration around non-necrotic fibers, rimmed vacuoles, and cytoplasmic positivity for MHC-I expression. However, these findings are not always specific for sIBM. As a new diagnostic tool, we first evaluated anti-cN1A positivity in muscle specimens obtained from Japanese patients with sIBM in this study. Anti-cN1A antibodies were first reported in 2011 to target a 43-kDa protein in the serum of patients with sIBM [10]. Subsequent studies demonstrated that anti-cN1A antibodies have a sensitivity of 34% to 70% and a specificity of higher than 90% for sIBM [3,10-13]. Larman et al. first described the reactivity of these antibodies in human skeletal muscle and demonstrated the accumulation of cN1A reactivity in perinuclear regions and vacuole rims [3]. In our study, 86% of cases showed anti-cN1A positivity in these regions. This sensitivity is as high as that of other diagnostic findings of sIBM, such as the presence of rimmed vacuoles and endomysial cellular infiltration. As for the clinical features such as age, disease duration, serum CK level, complications, and treatment efficacy, there were no significant differences between cN1A reactive patients and nonreactive patients. Interestingly, anti-cN1A positivity was significantly lower in other types of inflammatory myopathy and morphologically normal control patients than in sIBM, which may suggest that the expression of cN1A reflects specific mechanisms of sIBM, such as myonuclear degeneration [3]. We therefore suppose that anti-cN1A positivity in skeletal muscle is very useful for the diagnosis of sIBM, even in the absence of rimmed vacuoles or cellular infiltration. As compared with previous reports [3], there may be no racial differences in the usefulness of anticN1A antibodies for the diagnosis of sIBM (Figure 2).
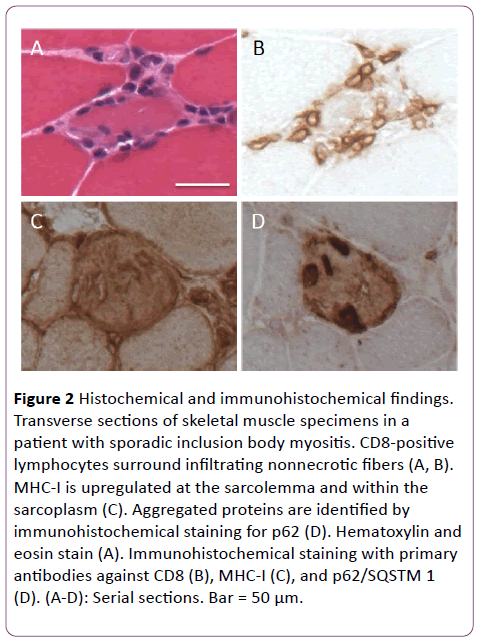
Figure 2: Histochemical and immunohistochemical findings. Transverse sections of skeletal muscle specimens in a patient with sporadic inclusion body myositis. CD8-positive lymphocytes surround infiltrating nonnecrotic fibers (A, B). MHC-I is upregulated at the sarcolemma and within the sarcoplasm (C). Aggregated proteins are identified by immunohistochemical staining for p62 (D). Hematoxylin and eosin stain (A). Immunohistochemical staining with primary antibodies against CD8 (B), MHC-I (C), and p62/SQSTM 1 (D). (A-D): Serial sections. Bar = 50 μm.
As for the clinical characteristics of Japanese patients with sIBM in this study, we found that concurrent heart disease and hepatitis C virus infection were more prevalent than reported previously. Although cardiac involvement may lead to fatal outcomes, the association of heart diseases with sIBM has rarely been reported previously [14]. Four of our patients with sIBM had heart disease: 3 had arrhythmias, and 1 had dilated cardiomyopathy. One of these patients died of dilated cardiomyopathy, but heart disease was not assessed histologically. Indeed, cardiac diseases often develop in elderly persons. However, given the risk of mortality, the prevalence of cardiomyopathy in patients with sIBM should not be neglected. Thus far, sIBM has been reported to be associated with hepatitis C virus (HCV) infection [15], human T-cell leukemia virus type I (HTLV-I) [16], or heart disease [14]. Recently, patients with sIBM have been reported to have HCV antibodies more commonly than the general population in Japan. We have also shown that the prevalence of HCV infection is higher in patients with sIBM than in the agematched general Japanese population (15% vs. 3.4%) [15]. On the other hand, only one patient concurrently had HTLV-I infection, and he came from a region endemic for HTLV-I. This coincidence may not have a great deal to do with the development of sIBM. Other clinical features of sIBM, such as male predominance, onset age, and the most common initial symptoms, were consistent with previous reports from Western countries or Japan [17,18]. As for treatment, corticosteroids and IVIg were ineffective for radical treatment, as previous studies have reported. Fewer patients received these medications in our study than in previous studies. This might have been attributed to the treatment-resistance of sIBM and concern about the side effects of immunosuppressants. The present study is limited by the relatively small sample size and by potential selection bias. Larger groups of patients must be assessed to better define the etiology of sIBM and the clinical significance of cN1A reactivity.
Conclusion
We found that sIBM developed in elderly persons, was more common in men than in women, and often presented with lower-limb weakness as the initial symptom in this series of Japanese patients. These findings are consistent with the results of previous studies in Western countries, but the concurrent presence of HCV infection and heart disease was remarkable in our study group. Pathologically, anti-cN1A antibodies were as highly positive as rimmed vacuoles or cellular infiltration in muscle biopsy specimens obtained from patients with sIBM. Evaluation of positivity for anti-cN1A antibodies in muscle tissue is considered a useful tool for the diagnosis of sIBM.
Consent
All patients gave fully informed consent before participation. This study was approved by the Ethics Committee of Nara Medical University School of Medicine.
Availability of Data and Materials
We agree to provide our dataset supporting the conclusions of this article.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
NE and KS conceived this original research article. KK, HN, NO, NI, RS, TK, TI, HK, and SU contributed to running the study and interpreted the data. NE and KS authored the manuscript, while the other authors read and commented on previous drafts. All authors read and approved the final manuscript.
Acknowledgements
We thank Ms Harumi Ikeda for her technical assistance.
17482
References
- Needham M, Mastaglia FL (2007) Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol 6: 620-631.
- Suzuki N, Aoki M, Mori-Yoshimura M, Hayashi YK, Nonaka I, et al. (2012) Increase in number of sporadic inclusion body myositis (sIBM) in Japan. J Neurol 259: 554-556.
- Larman HB, Salajegheh M, Nazareno R, Lam T, Sauld J, et al. (2013) Cytosolic 5′?nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol 73: 408-418.
- Rose MR (2013) 188th ENMC International Workshop: Inclusion Body Myositis 2-4 December 2011, Naarden, The Netherlands. NeuromusculDisord 23: 1044-1055.
- Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, et al. (1995) Inclusion body myositis and myopathies. Ann Neurol 38: 705-713.
- Hilton-Jones D, Miller A, Parton M, Holton J, Sewry C, et al. (2010) Inclusion body myositis: MRC centre for neuromuscular diseases, IBM workshop, London, 13 June 2008. NeuromusculDisord 20: 142-147.
- Hunsucker SA, Spychala J, Mitchell BS (2001) Human Cytosolic 5’-Nucleotidase I: Characterization and role in nucleoside analog resistance. J BiolChem 276: 10498-10504.
- Dubowitz V, Sewry C A, Oldfors A (2013) Muscle Biopsy: A Practical Approach. 4th edition. Saunders Elsevier, China
- Hoogendijk JE, Amato AA, Leckey BR, Choy EH, Lundberg IE, et al. (2004) 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. NeuromusculDisord 14: 337-345.
- Salajegheh M, Lam T, Greenberg SA (2011) Autoantibodies against a 43 KDa muscle protein in inclusion body myositis. PLoSONE 6: e20266.
- Herbert MK, Stammen VJ, Verbeek MM, Rietveld A, Lundberg IE, et al. (2016) Disease specificity of autoantibodies to cytosolic 5'-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann Rheum Dis 75: 696-701
- Pluk H, van Hoeve BJ, van Dooren SH, Stammen VJ, van der Heijden A, et al. (2013) Autoantibodies to cytosolic 5’-nucleotidase 1A in inclusion body myositis. Ann Neurol 73: 397-407.
- Greenberg SA (2014) Cytoplasmic 5’-nucleotidase autoantibodies in inclusion body myositis: Isotypes and diagnostic utility. Muscle Nerve 50: 488-492
- Utz W, Schmidt S, Schulz MJ, Luft F, Spuler S (2010) Cardiac involvement in sporadic inclusion-body myositis. Circulation 121: 706-708.
- Uruha A, Noguchi S, Hayashi YK, Tsurubara RS, Yonekawa T, et al. (2016) Hepatitis C virus infection in inclusion body myositis: A case-control study. Neurology 86: 211-217.
- Matsuura E, Umehara F, Nose H, Higuchi I, Matsuoka E, et al. (2008)?Inclusion body myositis associated with human T-lymphotropic virus-type I infection: eleven patients from an endemic area in Japan. J NeuropatholExpNeurol 67: 41-49.
- Benveniste O, Guiguet M, Freebody J, Dubourg O, Squier W, et al. (2011) Long-term observational study of sporadic inclusion body myositis. Brain 134: 3176-3184.
- Nakanishi H, Koike H, Matsuo K, Tanaka F, Noda T, et al. (2016) Demographic features of Japanese Patients with sporadic inclusion myositis: A single center referral experience. Intern Med 52: 333-337.







