Keywords
Buchholzia coriacea; Salmonella spp; Escherichia coli; Seed exract; Uropathogens
Introduction
Medicinal plants are a source of great economic value in the world of herbal medicine and is still the mainstay of about 75-80% of the whole population, mainly in developing countries for primary health care because of better cultural acceptability, better compatibility with the human body, and fewer side effects. The plant Buchholzia coriacea was named after R. W. Buchholz who collected plants in Cameroon in the late 19th century [1].
It belongs to the Capparaceae family. The seed of B. coriacea has medicinal values and these seeds gave the plants its common name of “wonderful kola” because of its usage in traditional medicine. The seeds are covered in a purple aril which is chewed in Ivory Coast and has a sharp pungent taste. Burkill [2] carried out a research on the medical uses of this plant in different parts of Africa and it has been used for years to treat a variety of illnesses; since it has been used continually over many generations it is likely that wonderful kola actually has an effect against pathogenic bacteria. Medicinal plants are believed to be important sources of new chemical substances with potential therapeutic effects. The secondary metabolites of plants have been found to be source of various phytochemicals that could be directly used as intermediates to produce new drugs. Traditional medicine should be able to play an even greater role in the modern primary healthcare system of developing countries [3]. Natural medicines are believed to be more acceptable to the human body, when compared to modern synthetic drugs. Thus, the most important factor needed is to derive the maximum benefit from the traditional system of medicine for providing adequate healthcare services to rural people [4]. Nature has long been an important source of medicinal agents. An impressive number of modern drugs have been isolated or derived from natural source, based on their use in traditional medicine [5].
Plants have been used traditionally for centuries and modern scientific studies have shown the existence of good correlation between the traditional or folkloric application of some of the plants further strengthens the search for pharmacological active components from plants [6]. Antibiotic resistance has become a global concern [7]. The Clinical efficacy of many existing antibiotics is being threatened by the emergence of multidrug-resistant pathogens. Many infectious diseases have been known to be treated with herbal remedies throughout the history of mankind [3]. The increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogenic microbial infectious agents has led to the screening of several medicinal plants for their potential antimicrobial activity [8].
Natural products, either as pure compounds or as standardized plant extract, provide unlimited opportunities for new drug leads because of the unmatched availability of chemical diversity. There is a continuous and urgent need to discover new antimicrobial compounds with diverse chemical structures and novel mechanisms of action for new and reemerging infectious diseases [7,9]. Therefore, researchers are increasingly turning their attention to folk medicine, looking for new leads to develop better drugs against microbial infections [10]. Considering the vast potentiality of plants as sources of antimicrobial agents, this study was undertaken to investigate the efficacy of ethanol and ethyl acetate seed extracts of Buchholzia coriacea against multidrug-resistant uropathogens so as to determine if it can serve as an alternative source of antimicrobial therapy.
Material and Methods
Study area
This study was carried out in Federal Teaching Hospital Abakaliki. FETHA is a tertiary hospital located in Abakaliki- Enugu express way. Ebonyi state is in the South East geopolitical zone of Nigeria, and it was created from Abia and Enugu States on 1st October, 1996. It derived its name from the Ebonyi River. The state capital of Ebonyi state is Abakaliki. The 2006 population census conducted in Nigeria pegged the population of Ebonyi state at an estimated population of 4.3 million people comprising 1,064,156 males and 1,112,791 females. The land mass of Ebonyi state is 5,935 km2. Approximately 75% of the population of Ebonyi state dwells in rural areas and the state is known for its farming activities. Salt, rice, yam, garri and solid minerals are some of the major produce and natural resources for which Ebonyi state is notable for [11].
Collection and re-characterization of bacterial isolates
One hundred and sixteen (116) clinical bacteria isolates comprising of Salmonella spp. (36) and E. coli (80) from UTI patients were collected from the Laboratory unit of Federal Teaching Hospital Abakaliki. Bacterial isolates collected from laboratory unit of FETHA were re-characterized using standard microbiology techniques [12].
Antibiotic susceptibility test
Antibiotic susceptibility of the isolates was determined using the Kirby-Bauer disc diffusion method according to the recommendations of the Clinical and Laboratory Standard Institute CLSI [13]. Isolates were sub-cultured on nutrient agar and incubated at 37?C for 24 hours. Then the colonies of each of the isolate was adjusted to 0.5 McFarland turbidity standard (equivalent to 1.5 × 108 cfu/ml). The standardized broth culture was incubated for 10 minutes. Using sterile swab stick, the standardized broth culture of the isolates was inoculated onto Mueller-Hinton agar plates. The surface of the medium was streaked in four directions while the plates were rotated approximately 60?C to ensure even distribution. The inoculated Mueller-Hinton agar plates could dry for 25 minutes.
Sterilized forceps were used to place the antibiotic discs evenly on the inoculated Mueller-Hinton agar so that the disc should be about 15 mm from the edge of the plate and not closer than 25 mm from disc to disc. After 30 minutes, the plates were inverted and incubated for 24 hours. A ruler was used to measure the diameter of each zone of inhibition in mm on the underside of the plate. The inhibitory zone diameters were interpreted as susceptible or resistant according to the criteria of CLSI (2015). The following standard antibiotic discs were used against the isolates; ampicillin (AMP, 10 μg), cefotaxime (CTX, 30 μg), ceftazidime (CAZ, 30 μg), gentamicin (GEN, 10 μg), ciprofloxacin (CIP, 5 μg), imipenem (IPM,10 μg), Ceftriaxone (CRO, 30 μg), Cefuroxime (30 μg), ofloxacin (10 μg), aztreonam (30 μg), ertapenem (ETP, 10 μg), nitrofurantoin (N, 100 μg) (Oxoid, Uk).
Multiple antibiotic resistance (MAR) index The multiple antibiotic resistance index was calculated as the ratio of the number of antibiotics to which the isolates were resistant/the total number of antibiotics against which the isolates were tested [14].
Collection of Buchholzia coriacea seeds
Seeds of Buchholzia coriacea were collected from Benin City. Seed samples were thoroughly washed with water, dried, and blended into powder form before experimental use.
Preparation of ethanol and ethyl acetate extracts of Buchholzia coriacea seeds
Exactly 20 grams of Buchholzia coriacea seed was weighed and separately added to 200 ml of 75% concentrations of ethanol and ethyl acetate in conical flask and were left to stand undisturbed for 24 hr with intermittent shaking. Extracts were then filtered using Whattsman no 1 filter papers and were allowed to air dry at room temperature. The dried extracts were collected using sterile container and were stored in the refrigerator at 4 0C pending the need for antibacterial assay [13]. Screening for the antibacterial activity of ethyl acetate and ethanolic seed extracts of Buchholzia coriacea against multidrug-resistant Salmonella spp. and Escherichia coli.
Exactly 20 mL of sterilized molten Mueller-Hinton agar was aseptically poured into sterile Petri-dish and then allowed to solidify. The prepared Mueller-Hinton agar plates were then streaked with standardized inoculums of the test bacteria that were adjusted to 0.5 McFarland turbidity standards. Thereafter, a sterilized 6 mm cork borer was used to bore 5 holes on the Mueller-Hinton agar plates and 4 of the holes were filled with equal volumes of ethyl acetate and ethanol seed extract that were diluted with 0.5% Dimethyl sulphoxide (DMSO). Ciprofloxacin (5 μg) disc was used as a positive control. The plates were allowed for about 30 mins for prediffusion of the plant extracts and was incubated at 37?C for 24 h. After incubation, the inhibition zone diameters were measured in millimeters using a meter rule. The inhibition zone diameter (IZD) of each plant extracts were evaluated by subtracting the size of the cork borer from the IZD measured.
Determination of minimum inhibitory concentration (MIC) of ethyl acetate and ethanolic seeds extracts of Buchholzia coriacea.
Varying concentrations of ethanolic and methanolic seed extracts of Buchholzia coriacea were re-constituted using twofold serial dilution method with sterile water. Varying concentrations which includes 100 μg/ml, 50 μg/ml, 25 μg/ml, 12.5 μg/ml, 6.25 μg/ml, and 3.125 μg/ml were prepared. The surface of the Mueller-Hinton agar plates were streaked with standardized inoculums of test organisms that was adjusted to 0.5 McFarland turbidity standards. Thereafter, a sterilized 6 mm cork borer was used to bore 5 holes on the Mueller- Hinton agar plates and 4 of the holes were filled with equal volumes of the different concentrations of plant extracts as diluted. The plates were incubated at 37?C for 18-24 h. Plates with clear zones of inhibitions were observed and recorded. Sterilized distilled water was used as the negative control while ciprofloxacin (5 μg) was used as the positive control.
Determination of the minimum bacteria concentration (MBC) of ethanolic and methanolic seeds extracts of Buchholzia coriacea
To determine the MBC, the plate of the MIC with clear zones of inhibitions were selected; isolates were taken from it, inoculated onto freshly prepared Mueller-Hinton agar and incubated at 37?C for 18-24 h. After incubation, extract concentration without microbial growth was taken as the MBC [15].
Phytochemical analysis of seed extracts of Buchholzia coriacea
Test for tannins: Five grams (5 g) of the seed extracts was stirred in 10 ml of distilled water and filtered. A few drops of ferric chloride were added to the filtrate. Presence of tannins was indicated by a blue-black, green or blue-green precipitate [16].
Test for saponins: One gram (1 g) of the extracts was mixed with 5 ml of distilled water. A few drops of Fehling’s solution were added to the extracts. Presence of green color indicated presence of saponins [17].
Test for flavonoid: Exactly 0.25 g of the seed extracts was each dissolved in 5 ml of ethanol. Thereafter, 0.6g of potassium hydroxide (KOH) was added to 20 ml of ethanol in a beaker and about 0.5 N of ethanol-potassium hydrogen was added to 1 ml of the filtrate. Appearance of yellow color indicated the presence of flavonoids [18].
Test for alkaloids: A 0.5g of the extract was stirred in 5 ml of aqueous hydrochloric acid on a steam bath (water bath). Thereafter, 1 ml of the filtrate was treated with a few drops of Mayers reagents, and production of turbidity from precipitation was taken as preliminary evidence for the presence of alkaloids [17].
Test for phenols: A 1 g of the extract was mixed with 2 ml of 2% solution of FeCl3. A blue-green or black coloration indicates the presence of phenols [16].
Test for anthocyanin: Exactly 2 ml of 2N HCL and NH3 was added to the extract. The appearance of a pink red color which turns to blue-violet indicated the presence of anthocyanin [17].
Test for terpenoids (Salkowski ’ s test): A 0.5 g of the individual extracts was added to chloroform and filtered. Acetic anhydride of about 10 drops was then added to the filtrate including 2 drops of concentrated Hydrogen Sulfide. A light green color at the interface indicated terpenes presence [19].
Results
A total of 116 clinical bacteria isolates comprising of Escherichia coli (80) and Salmonella species (36) from UTI patients were collected from microbiology laboratory unit of Federal Teaching hospital Abakaliki (FETHA). Out of the 80 E. coli isolates obtained from UTI patients, 35 were from males while 45 were from females. Contrastingly, out of the 36 Salmonella spp. isolates obtained from UTI patients, 12 were from males while 24 were from females.
Antibiotic susceptibility test results showed that ertapenem, meropenem, ofloxacin, and aztreonam were more active against E. coli and Salmonella species while ciprofloxacin, gentamicin, cefoxitin, cefuroxime, and ceftazidime were not active against the bacterial isolates (Figures 1-4). Results also showed that bacterial isolates were multidrug-resistant as they exhibited resistance to at least two different classes of antibiotics (Figures 1-4).
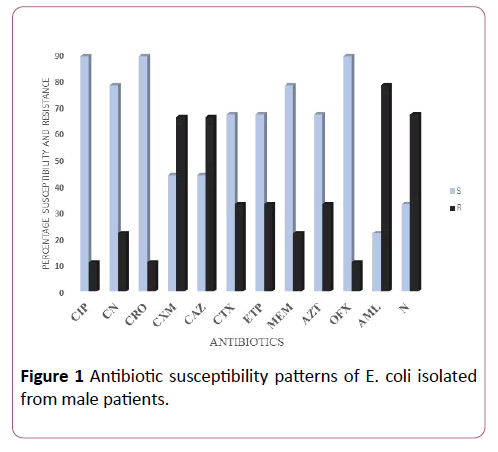
Figure 1: Antibiotic susceptibility patterns of E. coli isolated from male patients.
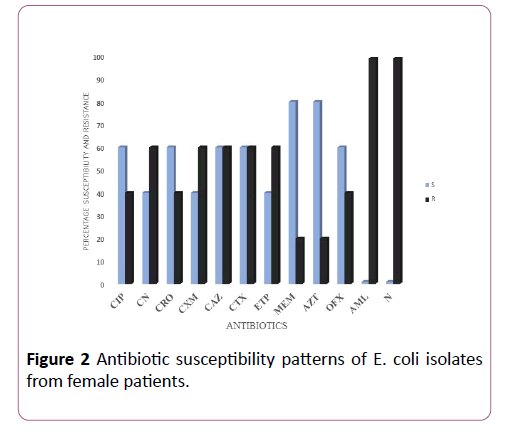
Figure 2: Antibiotic susceptibility patterns of E. coli isolates from female patients.
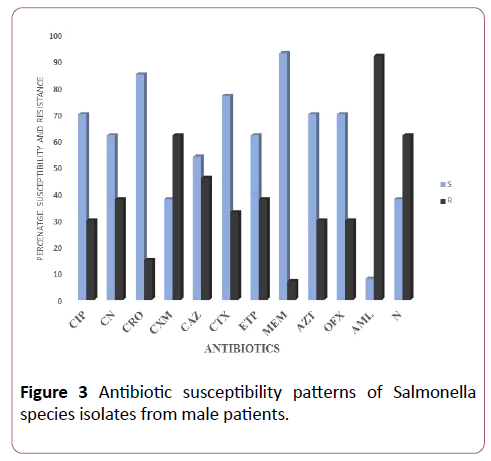
Figure 3: Antibiotic susceptibility patterns of Salmonella species isolates from male patients.
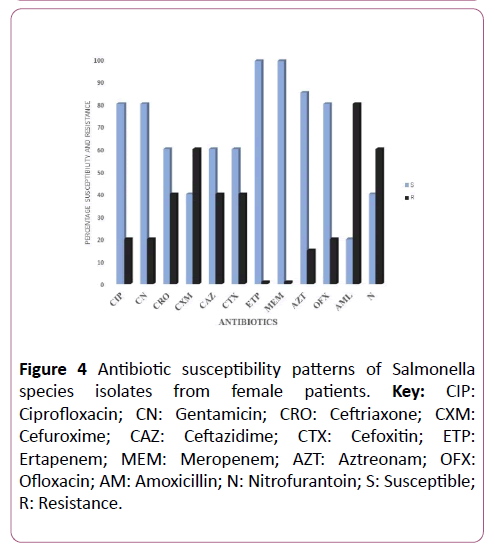
Figure 4: Antibiotic susceptibility patterns of Salmonella species isolates from female patients. Key: CIP: Ciprofloxacin; CN: Gentamicin; CRO: Ceftriaxone; CXM: Cefuroxime; CAZ: Ceftazidime; CTX: Cefoxitin; ETP: Ertapenem; MEM: Meropenem; AZT: Aztreonam; OFX: Ofloxacin; AM: Amoxicillin; N: Nitrofurantoin; S: Susceptible; R: Resistance.
Antibiotic susceptibility results also showed that there was no significant difference (p<0.05) in the antibiotic resistance and susceptibility frequencies of bacterial isolates from male patients when compared to female patients. Results of the antibacterial activities of ethanol and ethyl acetate seed extracts of Buchholzia coriacea showed no activity against all the uropathogens (E. coli and Salmonella species) obtained from FETHA (Tables 1-6).
Table 1 Multiple antibiotic resistance index (MARI) of E. coli collected from FETHA.
| Male |
SN |
Female |
| SN |
MARI value |
SN |
MARI value |
MARI value |
SN |
MARI value |
SN |
MARI value |
| 1 |
0.2 |
19 |
0.2 |
1 |
0.7 |
19 |
0.6 |
37 |
0.4 |
| 2 |
0.3 |
20 |
0.3 |
2 |
0.6 |
20 |
0.5 |
38 |
0.3 |
| 3 |
0.4 |
21 |
0.5 |
3 |
0.5 |
21 |
0.6 |
39 |
0.5 |
| 4 |
0.5 |
22 |
0.9 |
4 |
0.4 |
22 |
0.2 |
40 |
0.2 |
| 5 |
0.3 |
23 |
0.7 |
5 |
0.6 |
23 |
0.4 |
41 |
0.6 |
| 6 |
0.5 |
24 |
0.5 |
6 |
0.5 |
24 |
0.5 |
42 |
0.4 |
| 7 |
0 |
25 |
0.5 |
7 |
0.2 |
25 |
0.6 |
43 |
0.3 |
| 8 |
0.3 |
26 |
0.5 |
8 |
0.5 |
26 |
0.5 |
44 |
0.2 |
| 9 |
0.5 |
27 |
0.5 |
9 |
0.5 |
27 |
0.2 |
45 |
0.6 |
| 10 |
0.5 |
28 |
0.5 |
10 |
0.6 |
28 |
0.4 |
|
|
| 11 |
0.4 |
29 |
0.5 |
11 |
0.7 |
29 |
0.5 |
|
|
| 12 |
0.5 |
30 |
0.5 |
12 |
0.4 |
30 |
0.5 |
|
|
| 13 |
0.6 |
31 |
0.5 |
13 |
0.2 |
31 |
0.7 |
|
|
| 14 |
0.7 |
32 |
0.5 |
14 |
0.5 |
32 |
0.4 |
|
|
| 15 |
0.5 |
33 |
0.5 |
15 |
0.6 |
33 |
0.1 |
|
|
| 16 |
0.5 |
34 |
0.5 |
16 |
0.3 |
34 |
0.3 |
|
|
| 17 |
0.3 |
35 |
0.4 |
17 |
0.7 |
35 |
0.5 |
|
|
| 18 |
0.4 |
|
|
18 |
0.5 |
36 |
0.1 |
|
|
Key: SN=Serial Number, MARI: Multiple antibiotic resistance index
Table 2 Multiple antibiotic resistance index of Salmonella Species collected from FETHA.
| Male |
Female |
MARI value |
| SN |
MARI value |
SN |
MARI value |
SN |
| 1 |
0.7 |
1 |
0.7 |
19 |
0.6 |
| 2 |
0.3 |
2 |
0.6 |
20 |
0.5 |
| 3 |
0.6 |
3 |
0.8 |
21 |
0.2 |
| 4 |
0.5 |
4 |
0.4 |
22 |
0.6 |
| 5 |
0.6 |
5 |
0.9 |
23 |
0.4 |
| 6 |
0.5 |
6 |
0.5 |
24 |
0.6 |
| 7 |
0.7 |
7 |
0.2 |
|
|
| 8 |
0.3 |
8 |
0.5 |
|
|
| 9 |
0.8 |
9 |
0.8 |
|
|
| 10 |
0.5 |
10 |
0.6 |
|
|
| 11 |
0.4 |
11 |
0.4 |
|
|
| 12 |
0.8 |
12 |
0.4 |
|
|
| |
|
13 |
0.6 |
|
|
| |
|
14 |
0.8 |
|
|
| |
|
15 |
0.6 |
|
|
| |
|
16 |
0.7 |
|
|
| |
|
17 |
0.7 |
|
|
| |
|
18 |
0.9 |
|
|
Key: SN: Serial Number; MARI: Multiple antibiotic resistance index
Table 3 Inhibition Zone Diameter of Buchholzia coriacea ethanolic seed extracts against E. coli.
| Male |
|
Female |
| SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
| 1 |
NI |
19 |
NI |
1 |
NI |
19 |
NI |
37 |
NI |
| 2 |
NI |
20 |
NI |
2 |
NI |
20 |
NI |
38 |
NI |
| 3 |
NI |
21 |
NI |
3 |
NI |
21 |
NI |
39 |
NI |
| 4 |
NI |
22 |
NI |
4 |
NI |
22 |
NI |
40 |
NI |
| 5 |
NI |
23 |
NI |
5 |
NI |
23 |
NI |
41 |
NI |
| 6 |
NI |
24 |
NI |
6 |
NI |
24 |
NI |
42 |
NI |
| 7 |
NI |
25 |
NI |
7 |
NI |
25 |
NI |
43 |
NI |
| 8 |
NI |
26 |
NI |
8 |
NI |
26 |
NI |
44 |
NI |
| 9 |
NI |
27 |
NI |
9 |
NI |
27 |
NI |
45 |
NI |
| 10 |
NI |
28 |
NI |
10 |
NI |
28 |
NI |
|
|
| 11 |
NI |
29 |
NI |
11 |
NI |
29 |
NI |
|
|
| 12 |
NI |
30 |
NI |
12 |
NI |
30 |
NI |
|
|
| 13 |
NI |
31 |
NI |
13 |
NI |
31 |
NI |
|
|
| 14 |
NI |
32 |
NI |
14 |
NI |
32 |
NI |
|
|
| 15 |
NI |
33 |
NI |
15 |
NI |
33 |
NI |
|
|
| 16 |
NI |
34 |
NI |
16 |
NI |
34 |
NI |
|
|
| 17 |
NI |
35 |
NI |
17 |
NI |
35 |
NI |
|
|
| 18 |
NI |
|
|
18 |
NI |
36 |
NI |
|
|
Key: NI=No Inhibition
Table 4 Inhibition Zone Diameter of Buchholzia coriacea ethyl acetate seed extractsagainst E. coli
| Male |
|
Female |
| SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
| 1 |
NI |
19 |
NI |
1 |
NI |
19 |
NI |
37 |
NI |
| 2 |
NI |
20 |
NI |
2 |
NI |
20 |
NI |
38 |
NI |
| 3 |
NI |
21 |
NI |
3 |
NI |
21 |
NI |
39 |
NI |
| 4 |
NI |
22 |
NI |
4 |
NI |
22 |
NI |
40 |
NI |
| 5 |
NI |
23 |
NI |
5 |
NI |
23 |
NI |
41 |
NI |
| 6 |
NI |
24 |
NI |
6 |
NI |
24 |
NI |
42 |
NI |
| 7 |
NI |
25 |
NI |
7 |
NI |
25 |
NI |
43 |
NI |
| 8 |
NI |
26 |
NI |
8 |
NI |
26 |
NI |
44 |
NI |
| 9 |
NI |
27 |
NI |
9 |
NI |
27 |
NI |
45 |
NI |
| 10 |
NI |
28 |
NI |
10 |
NI |
28 |
NI |
|
|
| 11 |
NI |
29 |
NI |
11 |
NI |
29 |
NI |
|
|
| 12 |
NI |
30 |
NI |
12 |
NI |
30 |
NI |
|
|
| 13 |
NI |
31 |
NI |
13 |
NI |
31 |
NI |
|
|
| 14 |
NI |
32 |
NI |
14 |
NI |
32 |
NI |
|
|
| 15 |
NI |
33 |
NI |
15 |
NI |
33 |
NI |
|
|
| 16 |
NI |
34 |
NI |
16 |
NI |
34 |
NI |
|
|
| 17 |
NI |
35 |
NI |
17 |
NI |
35 |
NI |
|
|
| 18 |
NI |
|
|
18 |
NI |
36 |
NI |
|
|
Key: NI=No Inhibition
Table 5 Inhibition Zone Diameter of Buchholzia coriacea ethanolic seed extractsagainst Salmonella spp.
| Male |
Female |
| SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
| 1 |
NI |
1 |
NI |
19 |
NI |
| 2 |
NI |
2 |
NI |
20 |
NI |
| 3 |
NI |
3 |
NI |
21 |
NI |
| 4 |
NI |
4 |
NI |
22 |
NI |
| 5 |
NI |
5 |
NI |
23 |
NI |
| 6 |
NI |
6 |
NI |
24 |
NI |
| 7 |
NI |
7 |
NI |
|
|
| 8 |
NI |
8 |
NI |
|
|
| 9 |
NI |
9 |
NI |
|
|
| 10 |
NI |
10 |
NI |
|
|
| 11 |
NI |
11 |
NI |
|
|
| 12 |
NI |
12 |
NI |
|
|
| |
|
13 |
NI |
|
|
| |
|
14 |
NI |
|
|
| |
|
15 |
NI |
|
|
| |
|
16 |
NI |
|
|
| |
|
17 |
NI |
|
|
| |
|
18 |
NI |
|
|
Key: NI=No Inhibition
Table 6 Inhibition Zone Diameter of Buchholzia coriacea ethyl acetate seed extractsagainst Salmonella spp.
| Male |
Female |
| SN |
IZD (mm) |
SN |
IZD (mm) |
SN |
IZD (mm) |
| 1 |
NI |
1 |
NI |
19 |
NI |
| 2 |
NI |
2 |
NI |
20 |
NI |
| 3 |
NI |
3 |
NI |
21 |
NI |
| 4 |
NI |
4 |
NI |
22 |
NI |
| 5 |
NI |
5 |
NI |
23 |
NI |
| 6 |
NI |
6 |
NI |
24 |
NI |
| 7 |
NI |
7 |
NI |
|
|
| 8 |
NI |
8 |
NI |
|
|
| 9 |
NI |
9 |
NI |
|
|
| 10 |
NI |
10 |
NI |
|
|
| 11 |
NI |
11 |
NI |
|
|
| 12 |
NI |
12 |
NI |
|
|
| |
|
13 |
NI |
|
|
| |
|
14 |
NI |
|
|
| |
|
15 |
NI |
|
|
| |
|
16 |
NI |
|
|
| |
|
17 |
NI |
|
|
| |
|
18 |
NI |
|
|
Key: NI=No Inhibition
Minimum Inhibitory concentration (MIC) and Minimum Bactericidal Concentration of the Buchholzia coriacea seed extracts were not determined as they were inactive at all concentrations used against all the uropathogens (E. coli and Salmonella species). Phytochemical screening results of Buchholzia coriacea seed extracts showed the presence of some bioactive compounds such as tannins, saponins, phenol, flavonoids, alkaloids, and anthocyanins (Table 7).
Table 7 Phytochemical Properties of Seed Extracts of Buchholzia coriacea.
| Bioactive Compounds Screened |
Present |
| Saponin |
+ |
| Alkaloids |
+ |
| Flavonoids |
+ |
| Tannins |
+ |
| Phenolics |
+ |
| Anthocyanin |
+ |
Discussion
Recently, there has been considerable interest in the use of plant materials as alternative therapeutics to control pathogenic microorganisms [3]. The increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogens has led to the screening of several medicinal plants for their potential antimicrobial activity. This study was designed to determine the efficacy of ethanol and ethyl acetate seed extracts of Buchholzia coriacea (wonderful kola) against multidrug-resistant E. coli and Salmonella species isolates from urinary tract infections (UTIs) patients in microbiology laboratory unit of Federal Teaching Hospital (FETHA) Abakaliki. This study showed that E. coli and Salmonella spp were generally susceptible to ertapenem, imipenem, gentamycin, ceftriaxone, and aztreonam but resistant to amoxicillin and nitrofurantoin. Ethanol and ethyl acetate seed extracts of Buchholzia coriacea had no activity against all the test organisms. Phytochemical screening results indicated the presence of some bioactive compounds such as saponin, tannin, flavonoids, alkaloids, phenolics, and anthocyanins in Buchholzia coriacea. The frequency of distribution of bacterial isolates from urine samples of patients visiting FETHA in this study revealed that E. coli was the dominant bacterial pathogen. This study is in conformity with many other studies conducted in Nigeria. Our study agrees with the study by Abdul et al. [20], and Schnarr and Smaill, [21]. In their studies, E. coli was noted to be the predominant etiologic agent in both symptomatic and asymptomatic bacteriuria. Also, the study of Onoh et al. [22] indicated E. coli as the most prevalent pathogen. E. coli isolated amounted to 50.8% but this disagrees with the study conducted by Muazu, [23] in Kaduna and Iroha et al. [24] in Abakaliki, who reported Staphylococcus aureus as the predominant infecting organism in UTI respectively. These differences are because of the organisms of interest in our study as we did not include S. aureus. E. coli and Salmonella species were found to be highly resistant to ampicillin, nitrofurantoin, ceftazidime, cefuroxime, and gentamicin. Ideally, antimicrobial agent is expected to have inhibitory activities on almost all infectious pathogens implicated in an infection. Antibiotics susceptibility pattern of uropathogens can also vary with respect to individuals and environments. This might be as a result of self-medication practice due to access to over the counter availability of most antibiotics, non-amenability with prescriptions, sharing of suppository medicine by different individuals with similar symptoms and transfer of resistant isolates between people [25]. Drug resistance by pathogens is a serious health challenge because of the fast turnover and spread of mutant strains harbouring highly resistant genes. This could explain why antibiotics in the same class despite having the same functional group and binding sites, encounter varying sensitivity and resistance patterns. This was observed in our study with the carbapenems (imipenem, meropenem, and ertapenem), where imipenem had remarkably high activity on all isolates than ertapenem. In the present study, diverse susceptibility patterns of the bacterial pathogens were observed. The antibiotic with the highest activity were the carbapenems (imipenem, ertapenem, and meropenem). This is not worrisome due to the fact that these antibiotics are not readily available over the counter or even when it is available, it is usually very expensive (can only be affordable by few), and its administration route (parenteral) prevents its misuse and abuse especially in developing countries. Remarkable susceptibility to gentamicin was also observed among our isolates. Our result agrees with other studies by Idris et al. [26] in Ilorin; Iroha et al. [24] and Onoh et al. [22], both in Abakaliki, who also reported appreciable spectrum of activity with gentamicin. A worrisome resistance level to the third generation cephalosporins; ceftazidime, cefuroxime, and cefoxitin which are considered as drug of choice in pyelonephritis management and generally active in symptomatic urinary tract infection was noted. Though some strains were sensitive to the cephalosporins (ceftriaxone). This is similar and agrees with the work of Duft [27] and Onoh et al. [22] where sensitivity result showed 87.3% for cefpodoxime and 66.7% for ceftriaxone, but disagrees with the work of Idris et al. [26] in Ilorin, and Iroha et al. [24] in Abakaliki, where it was noted that the isolated uropathogens exhibited high resistance to third generation cephalosporins including ceftazidime, cefoxitin, cefotaxime, cefepime, cefazolin, and ceftriaxone. Nitrofurantoin and amoxicillin which are usually drugs of choice for the treatment of UTI were ineffective against the test bacterial pathogens (E. coli and Salmonella species) in our study. This could be due to the acquisition of multidrug resistance genes via genetic transfer mechanisms. This reflected in our present study where resistance of up to 90% were recorded for nitrofurantoin and amoxicillin respectively. This is in conformity with studies of Shittu and Mandara [28] in Zaria, who noted high sensitivity to gentamicin but resistance to amoxicillin and nitrofurantoin. It also agrees with the work of Idris et al. [26] at Ilorin teaching hospital but disagrees with the work of Okonko et al. [29] in Ibadan, where nitrofurantoin and nalidixic acid had significant activity against uropathogens. Ciprofloxacin, aztreonam, and ofloxacin showed relative sensitivity level with varying degrees. This agrees partly with the studies of Kolawole et al. [30] and Muazu [23], where uropathogenic bacteria exhibited high sensitivity to ciprofloxacin, ofloxacin, and nalidixic acid. The result of the multiple antibiotic resistance index (MARI) showed that the test bacterial pathogens used in this study had MARI values which ranged from 0.2 to 0.9. Since the standard is placed on a MARI value of 0.2, therefore MARI values greater than 0.2 was considered high risk source of contamination where antibiotics usage is often abused [31]. Our study showed that the test bacterial pathogens were multidrug-resistant as they exhibited resistance to at least two different classes of antibiotics. This further depicts the abuse of antibiotics in our study area. Nwinyi and Nduchukwuka [32] in their study reported that members of the Enterobacteriaceae isolated from patients in the hospital are multidrug-resistant in nature. Also, in line with the result of our study are the works of Khatib et al. [33] which were carried out in Lebanon and Mozambique respectively. They reported that enteric and non-enteric bacteria isolated from patients in the hospital are multidrug-resistant in nature especially to antibiotics in the beta-lactam group, aminoglycosides, and fluoroquinolones [33]. The increasing failure of available antibiotics and the impact of antibiotic resistance exhibited by pathogens has led to the screening of several medicinal plants for their potential antimicrobial activity. Plant-based products have been effectively proven for their utilization as source for antimicrobial compounds. Ethyl acetate and ethanol seed extracts of Buchholzia coriacea (wonderful kola) showed no activity against all tested bacterial pathogens. This shows that seed extracts of Buchholzia coriacea (wonderful kola) will not be a better naturally occurring therapeutic agent in treating UTIs as no zone of inhibition was shown by the seed extracts. The phytochemical constituents screening of ethyl acetate and ethanol seed extracts of Buchholzia coriacea showed significant photochemical constituents, such as saponin, tannin, flavonoids, alkaloids, phenolics, and anthocyanins in all the extraction solvents used. This is in line with the work of Bipul- Biswas et al. [9] who also reported the presence of these bioactive molecules. This present study showed that the ethyl acetate and ethanol seed extracts of Buchholzia coriacea have all the phytochemical constituent studied with negligible variation. Nevertheless, the significant presence of these bioactive components did not have any effect against E. coli and Salmonella spp. tested in this study. This might possibly be due to the multidrug-resistant traits expressed by the uropathogens.
Conclusion
Thus, this study revealed that ertapenem, imipenem, and gentamicin are still active against uropathogenic bacteria (E. coli and Salmonella species) while seed extracts of Buchholzia coriacea were ineffective and cannot be used as an alternative therapeutic choice in treating UTIs. Although, our study showed that seed extracts of Buchholzia coriacea were inactive against the uropathogens tested, they still contained significant secondary metabolites, such as saponin, tannin, flavonoids, alkaloids, phenolics, and anthocyanins, which are very beneficial to man's health for the prevention and management of diseases/infections. The use of plant and/or plant products for the treatment of infections and as alternative medications is an age-old custom in most of Nigerian homes. Notwithstanding, Buchholzia coriacea is not a good choice for treating infection caused by these organisms based on our findings. Also, there is a great need for man to utilize nature and embrace its treasures and panacea in form of herbs and spices to treat diseases. Awareness should also be carried out, especially in developing countries to curtail the trend in consumption of some acclaimed herbal plants/ medicinal plant until scientific study is carried out.
Conflict of Interest Statement
None to declare
28097
References
- Keay RWJ, Phil D, Biol FT (1989) Trees of Nigeria. Oxford University Press, New York. 204-207.
- Burkill HM (1995) The useful plants of West Tropical Africa Royal Botanic Gardens. London 3: 101.
- Nostro A, Cellini L, Bartolomeo S (2006) Effects of Combining extracts (From Propolis or Zingiberofficinale) with Clarithromycin on Helicobacter Pylori. Phytotherapyres 20: 187-190.
- Ghani A (1990) In traditional Medicine. Jahangirnagar University, Savar, Dhaka 15-40.
- Selvamohan T, Ramadas V, Shiba SS (2012) Antimicrobial Activity of Selected Medicinal Plants Against Some Selected Human Pathogenic Bacteria. Advance in Applied Science Research 3: 3374-3381.
- Egharevba HO, Kunl OF (2010) Preliminary Phytochemical Andproximate Analysis of the Leaves of Piliostigmathioniningii (Schumach) Mile-Redhead. Ethanobotanical Leaflets 14: 570-577.
- Gopalkrishnan, S, George S, Benny PJ (2010) Antimicrobial effect of Punicagrantum on Pyogenic Bacteria. Journal of Pharma and Biomedical Sciences 3: 342-351.
- Iwu MW, Duncan AR, Okunji CO (1999) New Antimicrobials of Plant Origin. Injanick J, 1st Ed. Perspectives on New Crops and New Uses, Alexanderia, VA: ASHS Press 457-462.
- Bipul B, Kimberly R, Fredrick M, Dwayne O (2013) Antimicrobial Activities of Leaf Extracts of Guava (Psidium guajava L.) on Two Gram-Negative and Gram-Positive Bacteria. International Journal of Microbiology 2: 231-234.
- Stainer RY, Ingraham JL, Wheelis ML (1986) General Microbiology 5th Ed. London. Themacmillan Press Ltd. 23
- Ezegwui HU, Onoh RC, Ikeako LC, Onyebuchi A, Umeorah O, et al. (2013) Investigating maternal mortality in a public Teaching Hospital, Abakaliki, Ebonyi State, Nigeria. Annual Medical Health Science Research 3: 75-80.
- Cheesbrough M (2006) District Laboratory Practice in Tropical Countries, Part 2. Cambridge University Press, Cambridge Press 137-157.
- Clinical and Laboratory Standards Institute (2015) Performance standards for antimicrobial susceptibility testing; Twenty-fifth informational supplement; M100-S25. Diversity of the human intestinal microbial flora. Science 308: 1635-1638.
- Moses IB, Ugbo EN, Odah EE, Iroha IR, Agumah NB, et al. (2018). Antibiogram and Phenotypic Characterization of E. coli Isolated from Nigeria’s Paper Currencies obtained from Butchers in Ebonyi State. Archives of Clinical Microbiology 9: 1-5.
- Jasmin AF, Md Faruk H, Aleya M (2017) Antibacterial Effects of Guava (Psidium gaujava L.) Extracts Against Food Borne Pathogens. International Journal of Nutrition and Food Sciences 6:11-15.
- Eleyinmi AF, Sporns P, Bressler DC (2008) Nutritional Composition of Gongronema latifolium and Vernonia amygdalina. Nutritional Food Science 38: 99-109.
- Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA (2005) Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. British Medical Council Complement. Alternative Medicine 5: 6-10.
- Tona L, Cimanga RK, Mesia K, Musuamba CT, De Bruyne T, et al. (2004) In vitro antiplasmodial activity of extracts and fractions of seven medicinal plants used in the Democratic Republic of Congo. Journal Ethnopharmacology 93: 27-32.
- Erasto P, Grierson DS, Afolayan AJ (2006) Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. Journal Ethnopharmacology 106: 117-120.
- Abdul IF, Onile BA (2001) Bacterial isolates from the urine of women in Ilorin and their antibiotic susceptibility patterns. Trop J Obstet Gynaecol 18: 61-65.
- Schnarr J, Smaill F (2008) Asymptomatic Bacteriuria and Symptomatic Urinary Tract Infections in Pregnancy. European Journal of Clinical Investigation 38: 50-57.
- Onoh RC, Umeora OUJ, Egwuatu VE, Ezeonu PO, Onoh TJP (2013) Antibiotics Sensitivity Pattern of Uropathogens from Pregnant Women with Urinary Tract Infection in Abakaliki, Nigeria. Infection and Drug Resistance 6: 225-233.
- Muazu M (2015) Urinary Tract Infections Amongst Pregnant Women Attending a Medical Centre in Kaduna, Nigeria. African Journal of Clinical and Experimental Microbiology 16: 7-11.
- Iroha I, Nwakeze E, Ejikeugwu C, Oji A, Udu-Ibiam E, et al. (2013) Frequency and Antibiogram of Uropathogens Isolated from Urine Samples of HIV Infected Patients on Antiretroviral Therapy. American Journal of Bioscience 1: 50-53.
- Vazquez JC, Villar J (2003) Treatment for Symptomatic Urinary Tract Infections During Pregnancy (Cochrane Review). Cochrane Database of Systematic Reviews, Issue 4. Chichester, UK: John Wiley and Sons Ltd.
- Idris S, Foltys V, Tančin V, Kirchnerová K, Tančinová D, Zaujec K (2014) Mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Nitra, Slovakia. Slov J Anim Sci 47: 33-38.
- Duft P (2002) Antibiotic Selection in Obstetric: Making Cost Effective Choices. Clinical Obstetrics and Gynaecology 45: 59-72.
- Shittu SO, Mandere MU (1999) Asymotomatic bacteriuria in antenatal patients in A.U.TH Zaria. Trop J Obst Gynea 16: 41-41.
- Okonko IO, Ijandipe LA, Ilusanya OA, Donbraye-Emmanuel OB, Ejembi J, et al. (2009) Incidence of Urinary Tract Infection (UTI) Among Pregnant Women in Ibadan, South-Western Nigeria. African Journal of Biotechnology 8: 6649-6657.
- Kolawole AS, Kolawole OM, Kandaki-Olukemi YT, Babatunde SK, Durowade KA et al. (2009). Prevalence of Urinary Tract Infection (UTI) Among Patients Attending Dalhatu Araf Specialist Hospital, Lafia, Nasarawa State, Nigeria. International Journal of Medicine and Medical Sciences 1: 163-167.
- Osundiya OO, Oladele RO, Oduyebo OO (2013) Multiple antibiotic resistance (MAR) indices of Pseudomonas and Klebsiella species isolates in Lagos university teaching hospital. African Journal Experimental Microbiology 14: 164-168.
- Nwinyi OC, Nduchukwuka D (2016) Antibiogram of Bacteria Species Isolated from Vegetables in Ado-Odo Ota, Nigeria. Journal of Biological Sciences 16: 188-196.
- Khatib A, Olama Z, Khawaja G (2014) Isolation, purification, and identification of some multi-drug resistant food-borne pathogens in lebanese fresh produce. International Research Journal of Pure and Applied Physics 2: 28-41.









