Keywords
Endophytic fungi; Pinus roxburghii; Penicillium frequentans; Antimicrobial activity; Phytochemical analysis
Introduction
Fungi are associated with many crucial processes like decomposition, recycling and transportation of nutrients in different environments, therefore are important components in every ecosystem. It has been predicted that there are only a small fraction (approx. 5%) that have been identified among a million different fungal species on this Earth [1]. Plant endophytes not only consist of fungi but also bacteria which in most cases coexist with endophytic fungi.
In which, Endophytes are a rich and good source of genetic diversity and novel species. These are a large and diverse group of fungi which colonize healthy plant tissues without causing any symptoms [2]. This may help the product discovery processes. Endophytes protect plants against herbivore, insect attack or tissue invading pathogens and thus show mutualistic, parasitic and commensalitic relationship with its host [3]. In India to treat various ailments, people uses extract from many types of local plants in a traditional way [4,5]. A large percentage of endophytes have been generally isolated from trees since only small proportions of endophyes have been found in herbaceous plants and shrubs [6]. They produce a variety of secondary metabolites, which confer major ecological benefits to their host plants [7,8]. Many of those metabolites act as potential therapeutic agents against cancer and infectious diseases [9]. Bioactive compounds classified as alkaloids, terpenoids, steroids, quinones, phenylpropanoids, phenols and lactones [10,11]. Many scientists concluded that plant growing in tropical rain forest where there is competition for light and nutrients and are most probably the host for endophytes that make bioactive compounds. Recent study proved that endophytes from tropical regions produced much more bioactive secondary metabolites than those from temperate parts of the world [12]. Mostly the bioactive metabolites are produced by endophytic fungi which are the major source of drugs and the plant clearly provides the proper environment for its growth and survival. Indeed, since the discovery that the endophytic fungi isolated form Taxus brevifolia, T. celebica, T. mairei, T. chinensis var. mairei, and T. wallachiana produced the anticancer drug taxol, many researchers have studied the endophytic fungi of medicinal plants to name source of novel medicine [13-15]. For instance, the relationship between the Pacific yew and the endophytic fungus, an endophyte of the Pacific Yew tree synthesizes a powerful chemotherapeutic agent called taxol which is further used in the treatment of cancer [16]. In the present study, the endophytic fungi were isolated from living symptomless spikes of Pinus roxburghii and the fungal extracts were screened for their antimicrobial and phytochemical analysis.
Material and Methods
Collection of plant material
The Spikes were collected of Pinus roxburghii from hilly areas of Uttarakhand state namely Pauri, Garhwal region belonging to different altitudes in the Himalayan region. The plant material was brought to the laboratory in sterile bags and processed within a few hours after sampling. Fresh plant materials were used for isolation work to reduce the chance of contamination.
Isolation of endophytic fungi
Healthy spikes were thoroughly washed in running tap water, then surface sterilized by a modified method of [17]. The selected spike segments were immersed in 95% ethanol for 30 sec, 4% sodium hypochlorite solution for 3 min and 95% ethanol for 30 sec followed by rinsing with sterile distilled water three times and allowed to surface dry under sterile conditions. After drying, each spike segment was cut into approximately 0.5 cm and placed on petri plates containing potato dextrose agar medium (PDA) supplemented with streptomycin (100 mg/L) to suppress bacterial growth. Petri plates were sealed with parafilm and incubated at 30°C in a light chamber for up to one week. They were monitored every day for growth of endophytic fungal colonies. Fungi growing out from the samples were subsequently transferred onto fresh PDA plates to isolate pure colonies.
Morphological characteristics of isolated endophytic fungi
Sporulating fungi were identified based on colony morphology, conidiospore and conidiophore characteristics. The microscopic identification of the isolates was carried out by lacto phenol staining technique [18].
Fermentation and extraction
Secondary metabolites extraction method was carried out as described by with minor modification [19]. Endophytic fungal isolates were further inoculated into 250 ml Erlenmeyer flasks containing 100 ml Potato Dextrose Broth and incubated at room temperature for 21 days under stationary conditions with intermittent shaking. The broth culture was filtered to separate the mycelia and filtrate. After that equal volume of ethyl acetate was added to the filtrate. Mixed it well for 10 min and kept for 5 min till the two clear immiscible layers formed. The upper layer of ethyl acetate containing the extracted compounds was separated using separating funnel. The extract was concentrated by removing the solvents under reduced pressure at 35-40°C with a rotary evaporation. Extract is dissolved in DMSO and stored at 4°C.
Screening of Bioactive Properties of Fungal Metabolites
Screening the bioactivity of metabolites was performed by following antimicrobial assay. These fungal pathogenic cultures were obtained from Forest Reaserch Institute (FRI) Dehradun (UK) and bacterial pathogenic culture obtained from MTCC, Chandigarh.
Test organisms
Strains such as Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, Candida albicans, Rhizoctonia solani, Cladosporium herbarum were used to evaluate the antimicrobial activity.
Antibacterial activity
The agar plate diffusion assay method was used to evaluate the antimicrobial activity against the test microorganisms [20]. Sterile Mueller Hinton medium was poured aseptically and 100 μl (105 CFU/ml) of bacterial liquid culture in an exponential growth phase was spread onto the surface of plate. All the culture plates were allowed to dry for about 5 min. Wells were bored on the agar surface using a cork borer and filled with 200 μl of endophyte culture filtrates respectively. The next day the zone of inhibition were measured with a measuring scale and compared with the DMSO as control.
Antifungal activity
A 100 μl of fungal culture/spore was spread onto the surface of PDA. Immediately, 100 μl of crude extract was loaded onto the well. The fungal culture was incubated at 30ºC for 48-72 h and the zone of inhibition was recorded around the well.
Minimum inhibitory concentration
MIC was determined by the serial dilution method. Nutrient broth and Potato Dextrose broth was made and sterilized using autoclave. 1 ml of the prepared broth was dispensed into the test tubes labelled from 1 to 5 using sterile syringe and needle. A stock solution containing 10 μg/ml of the extract was prepared. Then 0.5 ml of the solution was dispensed into the tube 1. Subsequently, from tube 1 solution was serially transferred till tube 5 and 1 ml of the solution was discarded from it. Tube 6 was used as a control for sterility of the medium and tube 7 for viability of the organisms. An overnight culture of each of the test isolates was prepared in sterile nutrient broth and potato dextrose broth. 0.5 ml innoculum was transferred into each tube from tube 1 to tube 7 with exception of 6, to which another sterile broth was added. The final concentration of the extract in each of the test tubes numbered after dilution 10, 5, 2.5, 1.25 and 0.625 μg/ml were incubated at 37°C and 28°C for 24hr and examined for growth. The test tube in which growth failed to occur was the MIC of the culture.
Screening of Fungal Metabolites produced by Endophytic Fungi
Chemical prospecting in ethyl acetate extract of endophytic fungi were performed to observe the presence of eight groups of compounds already found as having anti-microbial activity, which can be present in the secondary metabolism of endophytic fungi [9]. They were checked for the presence of the following secondary metabolites such as alkaloids (Mayer reagents), saponins, terpenoids, flavonoids, steroids, phenols, and tannins (reaction with FeCl3) and were evaluated [21].
Preliminary qualitative phytochemical screening of fungal metabolites produced by endophytic fungi
Alkaloids: The fungal crude extract was dissolved in 2N HCL solutions. The mixture was treated with a few drops of Mayer’s reagent (3 ml of potassium iodide solution mixed with 2 ml mercuric chloride solution). The creamish precipitate indicates the presence of alkaloids.
Flavonoids: In the test tube, containing 1 ml of fungal crude extract a few drops of 20% NaOH solution was added. A change to yellow colour was observed which on addition of acid changed to colourless solution depicted the presence of flavonoids.
Phenols: The fungal extract was dissolved in 5 ml of distilled water. To this few drops of neutral 5% ferric chloride solution was added. A dark green colour indicated the presence of phenolic compounds.
Saponins: The presence of saponins was determined by frothing test. The fungal extract was vigorously shaken with distilled water and was allowed to stand for 10 min. Formation of a fairly stable emulsion indicated the presence of saponins.
Steroids: Libermann-Burchard reaction was performed to assess the presence of steroids. The crude fungal extract was added in 1 ml of chloroform solution. The mixture was treated with acetic anhydride and few drops of concentrated H2SO4 were added. A blue green ring indicated the presence of steroids.
Tannins: The fungal crude extract was treated with alcoholic FeCl3 reagent. A bluish black colour, which disappears on addition of a little dilute H2SO4, was followed by the formation of yellowish brown precipitate indicated the presence of tannins.
Terpenoids: 1 ml of fungal crude extract was mixed in 2 ml of chloroform. 3 ml of concentrated H2SO4 was then added to form a layer. A reddish-brown precipitate coloration at the interface formed indicated the presence of terpenoids.
Secondary Quantitative phytochemical screening of fungal metabolite produced by endophytic fungi
a) Determination of total Phenol content
Total phenolic content was estimated using the Folin-Ciocalteu colorimetric method described by with modification [22]. Briefly, the appropriate dilutions of the samples (0.2 ml) were oxidized with 0.5N Folin-Ciocalteu reagents for 4 min at room temperature. Then the reaction was neutralized with saturated sodium carbonate (75 g/L). The absorbance of the resulting blue color was measured at 765 nm after incubation for 2 hr at room temperature in dark with gallic acid taken as standard.
b) Determination of total flavonoid content
Total flavonoid content was determined by a colorimetric method reported by [23]. Extract samples (0.25 ml) at a concentration of 1 mg extract mL-1 were diluted with deionized water (1.25 mL). A sodium nitrite solution at 5% (0.75 ml) was added and samples were incubated for 6 min at room temperature. AlCl3 at 10% (0.15ml) was aggregated and the mixture was incubated (5 min). Finally, 0.5 ml of sodium hydroxide (1 M) was added. Made the volume of the mixture to 2.5 ml with distilled water and incubated at 25°C for 30 min. Absorbance was measured at 510 nm with quercetin as standard.
Result and Discussion
Sample collection and isolation of endophytic fungi
Out of seventeen, a total of four different endophytic fungal morphotypes were selected from spike of Pinus roxburghii by using Potato Dextrose Agar medium. These endophytic fungi were characterized morphotipically using lactophenole cotton blue using scotch tape technique (Data not shown). These isolates were identified by Forest Reaserch Institute (FRI), Deharadun as Alternaria alternata, Thielaviopsis basicola, Geotrichum albida, Penicillium frequentans.
The Majority of the recovered endophtytes belong to the Ascomycota. Fungal endophytes are especially common among the Ascomycota, representing at least five classes, dozens of families, and large numbers of previously unknown species [24- 26]. Only one species from the collected isolates in this study belong to the Dothideomycetes [27,28]. Endophytic fungus Pestalotiopsis sp. isolated from the leaves of Pinus caneriensis [29]. The results showed that Pinus roxburghii is a good source of endophytic fungi, since only one type of culture medium was used for the isolation process, and this unique method allowed the acquirement of a considerable number of endophytes. No microorganism had appeared from the last washing water, so the surface disinfection method was considered efficient.
Antimicrobial activity of endophytic fungus
The antimicrobial activity of isolated endophytic fungi tested against representative Gram positive and Gram negative bacteria, yeast and filamentous fungi by well diffusion method which has been reported in the (Table 1, Figure 1). Fungal extract of Alternaria alternate shows maximum inhibition zone 30 mm against S. typhimurium. A. alternate showed zone of inhibition of 25 mm, 22 mm, 15 mm against S. aureus, E. coli, C. albicans respectively (Figure 2). Fungal extract of P. frequentans showed zone of inhibition of 20.20 mm, 24.50 mm, 17 mm, 10 mm against S. aureus, E. coli, S. typhimurium, C. albicans respectively (Figure 3). Fungal extract of Thielaviopsis basicola showed zone of inhibition of 16 mm, 18 mm, 21.8 mm, 13 mm against S. aureus, E. coli, S. typhimurium, C. albicans (Figure 4). Fungal extract of Geotrichium albida showed zone of inhibition of 23 mm, 17 mm, 20 mm, 15.5 mm against S. aureus, E. coli, S. typhimurium, C. albicans respectively (Figure 5). Fungal extract of isolated endophytic fungal strains showed no zone of inhibition against pathogenic fungal such as R. solani, C. herbarum.
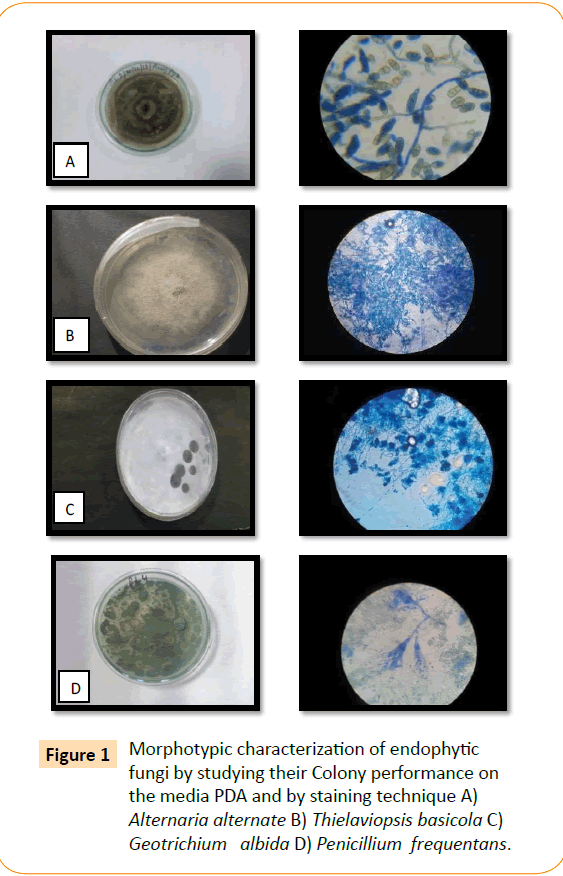
Figure 1: Morphotypic characterization of endophytic fungi by studying their Colony performance on the media PDA and by staining technique A) Alternaria alternate B) Thielaviopsis basicola C) Geotrichium albida D) Penicillium frequentans.
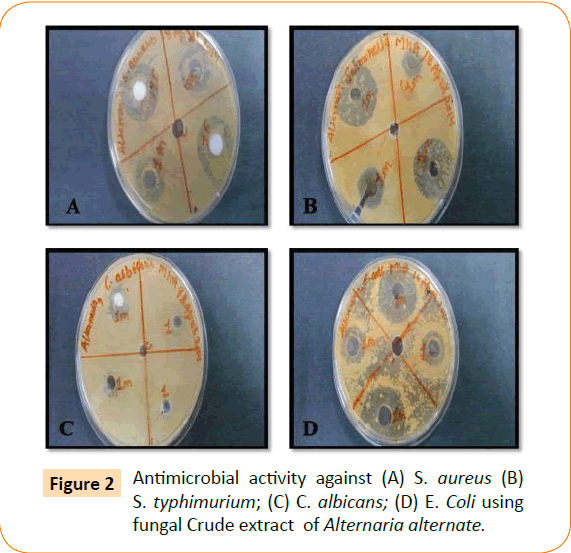
Figure 2: Antimicrobial activity against (A) S. aureus (B) S. typhimurium; (C) C. albicans; (D) E. Coli using fungal Crude extract of Alternaria alternate.
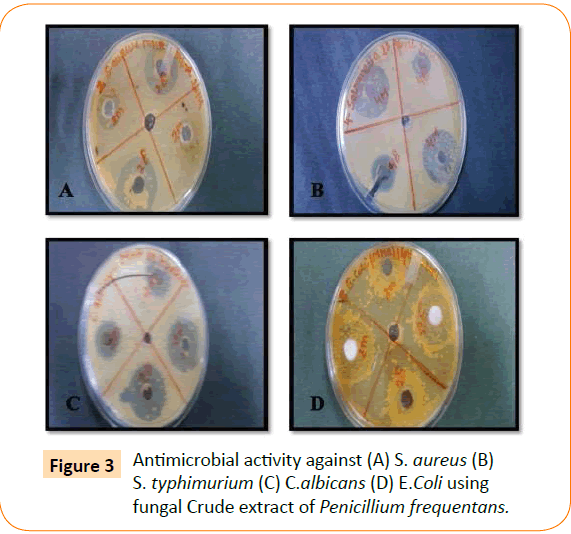
Figure 3: Antimicrobial activity against (A) S. aureus (B) S. typhimurium (C) C.albicans (D) E.Coli using fungal Crude extract of Penicillium frequentans.
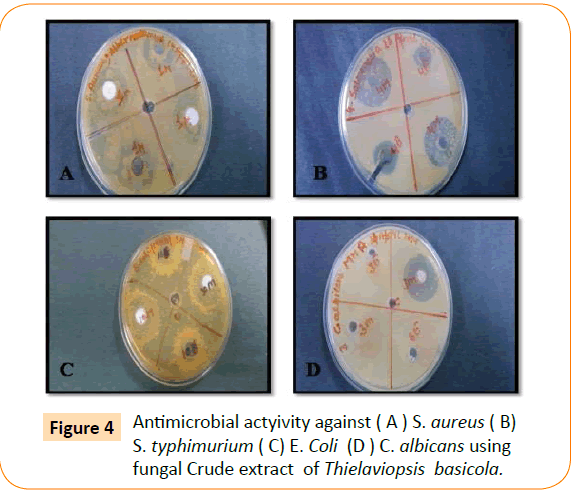
Figure 4: Antimicrobial actyivity against ( A ) S. aureus ( B) S. typhimurium ( C) E. Coli (D ) C. albicans using fungal Crude extract of Thielaviopsis basicola.
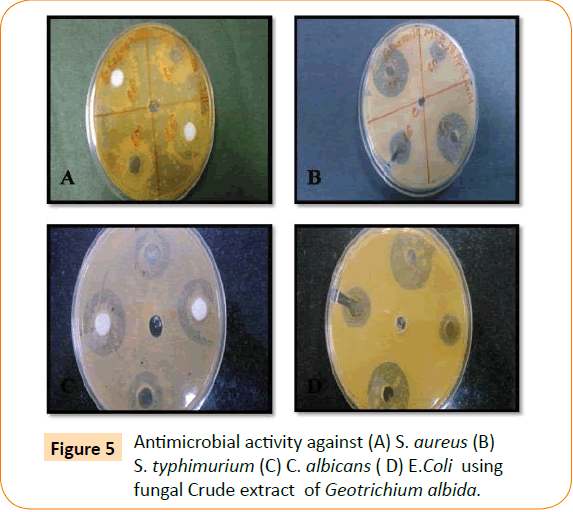
Figure 5: Antimicrobial activity against (A) S. aureus (B) S. typhimurium (C) C. albicans ( D) E.Coli using fungal Crude extract of Geotrichium albida.
| Endophytic fungal isolate |
Zone of inhibition by test organism (mm) |
| Endophytic Fungi |
SA |
EC |
ST |
CA |
| Alternaria alternata |
25.0 |
22.0 |
30.0 |
15.0 |
| Geotrichium albida |
23.0 |
17.0 |
20.0 |
15.5 |
| Penicilliumfrequentans |
20.2 |
24.5 |
17.0 |
10.0 |
| Thielaviopsis basicola |
16.0 |
18.0 |
21.8 |
13.0 |
Table 1: Antimicrobial activity of endophytic fungi isolated from Pinus roxburghii.
Endophytes are those organisms that colonize the living internal tissues of their hosts without causing detectable symptoms. Several fungal endophytes have been isolated from a variety of plant species which have proved as a rich source of secondary metabolites. In this present work a total of four fungal endophytes were isolated from Pinus roxburghii. Alternaria alternate, Penicillium frequentans, Thielavopsis basicola, Geotrichium albida was the main isolate and screened for the antimicrobial and production of secondary metabolites. Similarly, seven species of endophytic fungi were successfully isolated from Kigelia Africana and twelve endophytic fungi isolated from the healthy leaves of Madhucalongi folia L. for antimicrobial activity and the ethyl acetate extract of Colletotrichum gloeosporioides shown effective antimicrobial activity against S. aureus, E. coli and C. albicans [30,31]. The crude extract of fungal endophytes from Aegle marmelos, Plumbago zeylanica and Ficus carica that showed antibacterial activities against Pseudomonas aeruginosa [32]. The P. chrysogenum endophytic fungus isolated from Porteresia coarctata showed significant activity against Vibrio cholerae, a pathogen causing cholera in humans [33].
The need for new antimicrobial agents, in general, comes from the increasing rates of resistance to existing antibiotics. This problem extends beyond the clinical application of antimicrobial drugs, such as agricultural microorganisms are also known to have acquired resistance to commonly used antimicrobial chemicals. This may be due to partial extraction of bioactive compound by ethyl acetate, hexane, methanol etc. there is need for a suitable extracting solvent for the bioactive compounds extraction in this case.
Minimum Inhibitory Concentration
The minimum inhibitory concentration of isolated endophytic fungi tested against S. typhimurium, S. aureus, E. coli and C. albicans with a serial dilution tube method which has been reported in (Table 2). Penicillium frequentans and have shown MIC at lowest concentration 0.625 μg/ml against Bacillus subtilis. Geotrichium albida have shown MIC at lowest concentration 0.625 μg/ml against Salmonella typhii. Alternaria alternata have shown at 1.25 μg /ml against Bacillus subtilis. Thielaviopsis basicola have shown mic at 2.5 μg/ml against Bacillus subtilis and Candida albicans. Endophytic fungi isolated from Bacopa monnieri (L.) Pennell (Scrophulariaceae) showed MIC value 10– 100 μg/ml Minimum inhibitory concentration of the ethyl acetate extracts of endophytic fungi isolated from ethnomedicinal plants of the “Sacred forests” of Meghalaya, India against the test pathogens ranged from 13-45 μg/ml [34,35].
| S.No |
Isolates |
E.coli |
S. typhimurium |
B.subtilis |
C.albicans |
| 1 |
Alternaria alternata |
1.25 |
10.0 |
2.5 |
5.0 |
| 2 |
Geotrichium albida |
2.50 |
0.625 |
10.0 |
5.0 |
| 3 |
Penicilliumfrequentans |
1.25 |
2.5 |
0.625 |
10.0 |
| 4 |
Thielaviopsis basicola |
5.0 |
10.0 |
2.5 |
2.5 |
Table 2: MIC of endophytic fungus extracts against bacterial and fungal pathogen.
The crude extracts of Aspergillus sp. JPY1, Aspergillus sp. JPY2, Aspergillus niger and an unidentified sp. exhibited the maximum activity against three pathogenic Aspergilli sp. Their invitro minimum inhibitory concentrations (MICs) against Aspergilli were found to be 0.387 -12.50 mg/ml by micro-broth dilution [36]. In disc diffusion assay, only eleven out of fifty-one fungal extracts were found to be endowed with antimicrobial activity at a preset concentration of 50 μg/disc which could be the potential source to develop new antimicrobial agents.
Screening of fungal metabolites
In the past two decades, many valuable bioactive compounds with antimicrobial, antidiabetic, immune supressants, insecticidal, cytotoxic anticancer activities have been successfully discovered from the endophytic fungi. These bioactive compounds could be classified as alkaloids, terpenoids, steroids, quinones, lignans, phenols and lactones. Recently, endophytes are viewed as outstanding source of secondary metabolites and bioactive antimicrobial natural products. Similar to this the fungal metabolites analysis was carried out of the endophytic fungi showed the presence of saponins, flavonoids, tannins, and terpenoids. Fungal metabolites properties of crude extract of endophyte isolated from Pinus roxburghii plant shows that it has antimicrobial activity. This work requires further pharmacological screening for the isolation and identification of active compounds from the plant and its endophytic fungi.
Preliminary Qualitative Screening of fungal metabolite
The results of qualitative fungal metabolites analysis inferred that the fungal ethyl acetate extracts contain alkaloids, phenols, flavonoids, tannins and terpenoids. But steriods was absent in fungal extract. Saponins present only in one extract. This study was conducted to detect the presence of different fungal metabolites on the four recovered endophytic fungal isolate.
The preliminary screening of fungal metabolite compounds in ethyl acetate extract of endophytic fungi from Pinus roxburghii showed the presence of different fungal metabolite, phenolic compounds, steroids, tannins, alkaloids and flavonoids (Table 3). It shows the ability of endophytic fungi for providing antimicrobial activity. The endophytes has showed the presence of different phytochemicals such as, saponins, steroids, cardiac glycosides and tannins [37- 40]. Phytochemical analysis of crude extracts of endophytic fungi isolated from Plumeria acuminata L. and Plumeria obtusifolia L. revealed the presence of alkaloids, flavonoids, steroids, phenol and phenolic compounds [41]. Phytochemical screening of fungal endophytes acetonic, methanolic and water extracts isolated from Salvadora oleoides. Decne shown the presence of alkaloids, flavonoids, saponins, carbohydrates, tannins, sterols and terpenoids [42]. Phytochemical analysis revealed the presence of tannins, flavonoids, steroids, alkaloids, phenols and proteins from different solvents extracts of different endophytes isolated from Tabebuia argentea [43].
| Nameof fungal isolate |
Alkaloids |
Flavonoids |
Phenols |
Tannins |
Terpeniods |
Steriods |
Saponins |
| Alternaria alternate |
+ |
+ |
+ |
+ |
+ |
- |
- |
Thielaviopsis
basicola |
+ |
+ |
+ |
+ |
+ |
- |
- |
| Geotrichiumalbida, |
+ |
+ |
+ |
+ |
+ |
- |
+ |
| Penicilliumfrequentans |
+ |
+ |
+ |
+ |
+ |
- |
- |
Table 3: Qualitative determination of fungal metabolite of the selected Endophytic fungi.
The work on study of cytotoxicity and phytochemical screening (preliminary and GC-MS) of crude extracts of Aspergilus spp associated with Salvadora oleoides endowed with antimicrobial properties were investigated. Salvadora oleoides associated endophytic fungi could be an essential source of bioactive compounds useful for developing better antifungal or antibacterial drugs with good therapeutic index value [36].
Secondary Quantitative of Screening of Fungal Metabolite
Determination of total phenol content
The amount of total phenol was determined with the Folin- Ciocalteu reagent. Gallic acid was used as a standard compound and the total phenols were expressed as mg/g gallic acid equivalent using the standard standard curve equation: y = 0.0063x + 0.0394, R2= 0.9979, Where y is absorbance at 760 nm and x is total phenolic content in the extracts of fungal endophyte of Pinus roxburghii expressed in mg/gm. The maximum phenolic content was found in the ethyl acetate extract of Alternaria alternate (288.5 mg/g). Phenolic compounds are a class of antioxidant agents which act as free radical terminators and their bioactivities may be related to their abilities to chelate metals, inhibit lipoxygenase and scavenge free radicals [44].
The production of phenols and quinones by endophytic fungi and antimicrobial activity of these compounds are fairly known. Two new antimicrobial substances such as pestalachloride A and B were isolated, which are phenolic compounds produced by the endophytic fungus Pestalotiopsis adusta [45]. In another work, it was observed the production of quinones by Ampelomyces sp, an endophytic fungus isolated from Urospermum picroides, with significant activity against the pathogenic bacteria S. aureus, S. epidermidis and E. faecalis [46].
Determination of total flavonoid content
The amount of total flavonoid was determined with the Quercetin reagent. Quercetin was used as a standard compound and the total flavonoid were expressed as mg/g Quercetin equivalent using the standard curve equation: y = 0.1044x+0.0284, R2= 0.9999, Where y is absorbance at 540 nm and x is total flavonoid content in the extracts of different fungal metabolite expressed in mg/gm. Data in (Table 4) shows the contents of total flavonoid that were measured by AlCl3 reagent in terms of Quercetin acid equivalent. The total flavonoid varied from 17.41 to 17.48 mg/g in the extracts of all fungal endophytes. The maximum flavonoid content was found in the ethyl acetate extract of P. frequentans (17.48 mg/g). The results obtained from present study showed that the crude extract of P. frequentans also showed maximum presence of phenolic component (288.5mg/g) which contain highest amount of flavonoid and phenolic which can be used to explore new drugs. Ethyl acetate extraction is most efficient method of isolating fungal secondary metabolites. Ethyl acetate as an extraction solvent selectively extracts low molecular weight phenol and flavonoid.
| Endophytic fungi |
Total phenol (mg/g) |
Total flavonoid (mg/g) |
| Alternaria alternate |
288.14 |
17.41 |
| Thielaviopsisbasicola |
287.23 |
17.41 |
| Geotrichiumalbida |
286.77 |
17.4 |
| Penicilliumfrequentans |
288.34 |
17.48 |
Table 4: Total Phenolic and flavonoid content in different species of endophytic fungi.
Endophytic fungal extracts of ethyl acetate showed wide range of total phenolic concentrations varied from 4.20 to 60.13 mg GAE/g of dry weight [47]. The highest concentration of phenols was observed in extract of Chaetomium sp. (60.13±0.41 mg GAE) followed by Aspergillus niger (A. niger) strain. Whereas Fusarium sp., Curvularia lunata and Syncephalastrum racemosum extracts contained considerably least concentration of phenols.
Conclusion
Fungal endophytes from forest plants are under attention as these plants tend to produce natural products beneficial for us. A number of forests have been screened in different regions of world for endophytes. Still knowledge on comprehensive diversity of endophytic fungi from forest plant is scanty. Investigations have been carried on spikes of Pinus roxburghii plants. The present study provides evidence that isolated endophytes are capable to survive inside plants. Results of present study indicate that selected plants and their parts are highly colonized by microbial endophytes and endophytes are not host specific.
All endophytic fungi showed promising high antimicrobial activity against the bacterial and fungal pathogen. Endophytic fungi are a poorly investigated group of microorganisms that represent an abundant and dependable source of bioactive and chemically novel compounds with potential for exploitation in a wide variety of pharmaceutical and industrial areas. Some plants and their associated endophytes were found to produce the same natural compounds. Hence, the isolation of endophytic fungi from Pinus roxburghii for production of bioactive compound may facilitate the new product discovery process. However there is a need of further in-depth studies of these isolated endophytes. Further growing those on large scale, modifying culture and supplying some stimulants might help in getting better production of particular bioactive compound. This study suggested that the Pinus roxburghii is a great source of fungal endophytes.
Acknowledgment
We gratefully acknowledge TEQIP–II and G. B. Pant Engineering College, Pauri, Garhwal for financial support and providing instrumentation facilities.
6639
References
- Hawksworth, DL (2001)The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res105:1422-1432.
- PawÅ‚owska J,Wilk M, SliwiÅ, ska-Wyrzychowska A, et al. (2014) The diversity of endophytic fungi in the above-ground tissue of two Lycopodium species in Poland. Symbiosis 63: 87-97.
- Singh LP, Gill SS, Tuteja N (2011) Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav 6: 175-191.
- Jerlin BS,BrittoAJD, Balasingh J, SwamidossDP,Pralcash AA (2004) Medicinal flora of koonthakulam bird Sanctuary. J. Ecobiol16: 7-16.
- Rajasekara PM, Sharmila BG, Kumar G, Smila KH (2006) Medicinal Plants of ethanobotanical importance curing diabetes from Namakkal district (Tamilnadu), India. Indian Journal Environ. Eco-plan 12: 201-205.
- Strobel GA1 (2003) Endophytes as sources of bioactive products. Microbes Infect 5: 535-544.
- Kusari S,Hertweck C, Spiteller M (2012) Chemical ecology of endophytic fungi: origins of secondary metabolites. ChemBiol 19: 792-798.
- Kusari S, Pandey SP, Spiteller M (2013) Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry 91:81-87.
- Al AH, Debbab A, Kjer J,Proksch P (2010)Fungalendophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers41:1–16.
- Yu H, Zhang L, Li L, Zheng C, Guo L, et al. (2010) Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol Res 165: 437-449.
- Zhou L, Zhao J, Shan T, Cai X, Peng Y (2010) Spirobisnaphthalenes from fungi and their biological activities. Mini Rev Med Chem 10: 977-989.
- Bills G,Dombrowski A,Pelaez F,Polishook J, An Z (2002) Recent and Future Discoveries of Pharmacologically Active Metabolites from Tropical Fungi. In: Tropical Mycology, Watling R, JC Frankland, A.M. Ainsworth, S. Issac and C.H. Robinson (Eds.). CABI Publishing, New York 2:165-194.
- Lin X, Lu C, Huang Y,Zheng Z, Su W, et al. (2007)Endophytic fungi from a pharmaceutical plant, Camptothecaacuminata: isolation, identification and bioactivity. World J MicrobiolBiotechnol 23:1037–1040.
- De Siqueira VM, Conti R, De Araújo JM,Souza-Motta CM (2011)Endophytic fungi from the medicinal plant Lippiasidoides Cham and their antimicrobial activity. Symbiosis53:89-95.
- Kumaran RS, Kim HJ, Hur BK (2010) Taxol-producing [corrected] fungal endophyte, Pestalotiopsis species isolated from Taxuscuspidata. J BiosciBioeng 110: 541-546.
- Strobel G, Hess WM, Li JY, Ford E, Sears J, et al. (1997) Pestalotiopsisguepinii, a taxol producing endophyte of the Wollemi Pine, Wollemianobilis. A. J. of Bot 45:1073–1082.
- Raviraja NS1 (2005) Fungal endophytes in five medicinal plant species from Kudremukh Range, Western Ghats of India. J Basic Microbiol 45: 230-235.
- Nagamani A,Kunwar IK,Manoharachary C (2006) A Hand Book of Soil Fungi. New Delhi: I K international.
- Radji M, Sumiati A, Rachmayani R, Elya B (2011) Isolation of fungal endophytes from Garciniamangostana and their antibacterial activity. Afric J. Biotech 1:103-107.
- Hormazabal E,Piontelli E (2009)Endophytic fungi from chilean native gymnosperms: antimicrobial activity against human and phytopathogenic fungi. World J MicrobiolBiotechnol 25:813-819.
- Devi NN, Shankar DP,Sutha S (2012) Biomimetic synthesis of silver nanoparticles fromanendophytic fungus and their antimicrobial efficacy. Inter. J. biomed.Adv. Res: 409-415.
- Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74: 2157-2184.
- Chang C, Yang M, Wen H,Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Analaysis 10: 178-182
- Clay K (1989)Clavicipitaceousendophytes of grasses: their potential as biocontrol agents. Mycol. Res 92:1–12.
- Pepeljnjak S,Kosalec I, Kalodera Z, Blazević N (2005) Antimicrobial activity of juniper berry essential oil (Juniperuscommunis L, Cupressaceae). Acta Pharm 55: 417-422.
- Gehlot P,Bohra NK,Purohit DK (2008)EndophyticMycoflora of Inner Bark of Prosopis cineraria a Key Stone Tree Species of Indian Desert. Amer.-Eur J. Bot1: 01-04.
- Petrini O, Muller E (1979)PilzlicheEndophyten, am Beispiel von Juniperuscommunis L. Sydowia 32:224–251.
- Petrini O (1986) Taxonomy of endophytic fungi of aerial plant tissues. In: Microbiology of the Phyllosphere. (eds NJ Fokkema, van den Heuvel). Cambridge University Press, Cambridge 75–187.
- Bagyalakshmi, Thalavaipandian A, Ramesh V,Arivudainambi USE,Rajendran A (2012) A novel endophytic fungus Pestalotiopsis sp. Inhibiting Pinuscaneriensis with antibacterial and antifungal potential. International Journal of Advanced Life Sciences 1 : 1-7.
- Idris AM, Al-tahir I, Idris E (2013) Antibacterial activity of endophytic fungi extracts from the medicinal plant Kigeliaafricana. Science 5:1-9.
- Thalavaipandian A,Arivudainambi USE,Bagyalakshmi,Rajendran A (2011) Antimicrobial Potential of endophytic fungus Colletotrichumgloeosporioides associated with Madhucalongifolia L. Adv. Appl. Res 3:1-7.
- PrabavathyD,ValliNachiyarC (2012) Study on the antimicrobial activity of Aspergillus sp.isolated from Justiciaadathoda. Ind.J.Sci.Technol5:3317-3320.
- Devi P, Rodrigues C, Naik CG, D'Souza L (2012) Isolation and Characterization of Antibacterial Compound from a Mangrove-Endophytic Fungus, Penicilliumchrysogenum MTCC 5108. Indian J Microbiol 52: 617-623.
- Katoch M, Singh G, Sharma S, Gupta N, Sangwan PL, et al. (2014) Cytotoxic and antimicrobial activities of endophytic fungi isolated from Bacopamonnieri (L.) Pennell (Scrophulariaceae). BMC Complement Altern Med 14: 52.
- Bhagobaty RK, Joshi SR (2012) Antimicrobial and antioxidant activity of endophytic fungi isolated from ethnomedicinal plants of the “Sacred forests” of Meghalaya, India. MikologiaLekarska 19: 5-11.
- Dhankhar S,Yadav PJ (2013)Investigating Antimicrobial Properties of Endophytic fungi Associated with SalvadoraoleoidesDecne. Anti-Infective Agents 11:48-58.
- Khanna VG,Kannabiran K (2008) Antimicrobial activity of saponin fractions of the leaves of Gymnemasylvestre and Ecliptaprostrata. World J. Microbiol. Biotechnol 24: 2737-2740.
- Kalyoncu F,Oskay M, SaÄŸlam H, ErdoÄŸan TF, Tamer AU (2010) Antimicrobial and antioxidant activities of mycelia of 10 wild mushroom species. J Med Food 13: 415-419.
- Ahmad R, Ali AM, Israf DA, Ismail NH, Shaari K, et al. (2005) Antioxidant, radical-scavenging, anti-inflammatory, cytotoxic and antibacterial activities of methanolic extracts of some Hedyotis species. Life Sci 76: 1953-1964.
- Kaur R,Arora S (2009) Chemical constituents and biological activities of Chukrasiatabularis A. Juss. A Review. J. Med. Plants Research 3:196-216.
- Kamarian R,Ghasemlou F (2013) Screening of total phenol and flavonoid content, antioxidant and antibacterial activities of the methanolic extracts of three Silene species from Iran. Inter J. Agric. and Crop Sci. 5:305-312.
- Li E, Jiang L, Guo L, Zhang H, Che Y (2008) Pestalachlorides A-C, antifungal metabolites from the plant endophytic fungus Pestalotiopsisadusta. Bioorg Med Chem 16: 7894-7899.
- Aly AH,Edrada-Ebel R, Wray V, Müller WE, Kozytska S, et al. (2008) Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermumpicroides. Phytochemistry 69: 1716-1725.
- Ramesha A,Srinivas C. Antimicrobial activity and phytochemical analysis of crude extracts of endophytic fungi isolated from Plumeriaacuminata L. and Plumeriaobtusifolia L. Euro. J. Exp. Bio. 4:35-43.
- Dhankhar S, Kumar S,Dhankhar S,Yadav JP (2012) Antioxidant activity of fungal endophytes isolated from SalvadoraoleoidesDecne. Inter. J. of Pharm. Pharmaceutic. Sci4:380-385.
- Govindappa M, Channabasava R, Sunil RK,Pushpalatha KC(2013) Antioxidant activity and phytochemical screening of crude endophytes extracts of Tabebuiaargentea Bur. & K. Sch. Amer. J. Plant Sci4:1641-1652.
- Yadav M, Yadav A, Yadav JP (2014) In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac J Trop Med 7S1: S256-261.










