Goly Kouassi Roselin Cyrille1,2, Dadie Adjehi2*, Soro Yaya1, Kouame N’zebo Désiré2, Kassi Amian Brise Benjamin3 and DJE Marcellin2
1Laboratory for Industrial Processes of Synthesis, Environment and New Energies, National Polytechnique Félix Houphouët-Boigny Institute, Yamoussoukro, Côte d’Ivoire
2Department of Food Science and Technology, Laboratory of Biotechnology and Food Microbiology, Nangui Abrogoua University, Abidjan, Côte d'Ivoire.
3Department of chemistry, Laboratory of Organic Chemistry of Natural Substances, Félix Houphouëtboigny Cocody University, Abidjan, Côte d’Ivoire
*Corresponding Author:
Dadie Adjehi
Department of Food Science and Technology
Laboratory of Biotechnology and Food Microbiology
Nangui Abrogoua University, Abidjan
Côte d'Ivoire
Tel: 0022501879717
E-mail: thomasdadie@yahoo.fr
Received date: January 12, 2017; Accepted date: February 07, 2017; Published date: February 14, 2017
Citation: Goly KRC, Dadie A, Soro Y, Kouame ND, Kassi AB, et al. Antimicrobial and Preservative Activities of Lippia Multiflora Essential Oil on Smoked Mackerel (Scomber Scombrus) Fish. Arch Clin Microbiol. 2017, 8:1.
Copyright: © 2017 Adjehi D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Essential oil; Lippia multiflora; Mackerel; Scomber scombrus; Food preservation
Introduction
Fish is a very important source of animal protein in West Africa [1] but easily perishable if not properly preserved. This very perishable foodstuff after its capture [2] has an impact not only due to lack of food hygiene but also on social, medical and economical characteristics, due to socioeconomic constraints hampering the development of industrial preservation of fish [3]. Smoking is one of the several methods of preserving easilyperishable sea-foods, such as fish; however, this method, which is mostly done as cottage food processing method at the local settings, can be responsible for easier contamination of smoked fish by microbial pathogens [4]. Microbial contamination may be through food handling [5], and sometimes, inadequate of ineffective fish smocking can constitute a risk factor for consumers, especially, due to harmful effects of smoke [6,7]. Thus, smoked fish can be of public health importance because of presence of pathogenic microorganisms, as well as chemical contaminants [8].
Materials and Methods
Materials
The study was carried out on the smoked mackerel fish (Scomber scombrus) (Figure 1). In Côte d’Ivoire, mackerel is frequently consumed in rural and urban area because of its low cost, its taste and availability. It is eaten fresh (fresh mackerel) and smoked (smoked mackerel). But, because of the difficulties of conservation of fresh fish by rural and some urban people, they use smoked fish [9-11].

Figure 1: Smoked mackerel (Scomber scombrus) sample on selling site.
Methods
Choice criteria and Sampling: The study has been carried out over a period of 1 month (July 2014). A sample constituted of 3 smoked fish of 100 to 300 g was taken on smoking and selling outlets in Yamoussoukro metropolis (Côte d'Ivoire).
Smoked fish were collected on two types of sites. Some are located on open squares and others on closed squares [12,13]. All these sites are unsafe with household garbage, domestic animals, insanitary environment and women dressed in dirty clothes. Dirty equipment, unhygienic environment of smoking and inappropriate fish handling justified the choice. All Samples were collected in a sterile manner, kept in an ice box (ESKIMO), maintained at 4°C and transported to the laboratory for analysis. A total of 72 samples smoked mackerel were randomly and aseptically collected at a week. So, smoked fish from 0.5 to 1 kg were taken during the study.
Extraction of essential oil: Fresh leaves of L. multiflora were collected from July to September 2013 around the National Polytechnic Institute Félix HOUPHOUËT BOIGNY of Yamoussoukro (Côte d'Ivoire). After identification by a botanist of the institute, leaves in a Herbarium were stained in the sun at room temperature (27 ± 2°C) for ten days before using [14]. A quantity of 300 g of dry L. multiflora leaves was introduced in Clevenger apparatus for oils extraction. The extraction was performed by steam distillation in a Clevenger type apparatus [15]. After two hours and half the recovered essential oils are dried and then stored at 4°C in a hermetically sealed flask [16].
Microbiological quality of smoked fish: The microbiological quality of the samples was evaluated by the standards methods of microbiology. The Total Aerobic Mesophilic Flora (TAMF) at 30°C (NF V08-051), Total Coliform (NF ISO 4831) at 44°C, Staphylococcus aureus at 37°C (NF IN ISO 6888-1) and yeasts and moulds at 25°C (ISO 7954). The enumeration of each germ mentioned above was carried out according to standard NF V08-010. Indeed, ten gram (10 g) of smoked fish sample was weighed aseptically into 90 ml of peptone water to obtain the initial dilution of 10-1 then serial dilution was done. The Total Aerobic Mesophilic Flora (TAMF) was determined by plating 1ml of the serially diluted sample on Nutrient agar (Scharlau, Gato Perez, Spain) and incubating at 30 ± 1°C for 72 h. The presumptive Total Coliform (TC) counts were determined by plating 1 ml of the serially diluted sample on Violet Red Bile Lactose Agar (VRBL) (Ultimed, Castellar del Vallès, Spain) and incubating at 44 ± 1°C for 24 h. Fungi (yeasts and moulds) were determined by plating 0.1 ml of the serially diluted sample on Sabouraud (BIO-RAD; Marnes-la-Coquette; France) with Chloramphenicol and incubating at 25 ± 1°C for 72 h. Staphylococcus aureus was determined by plating 0.1 ml of the serially diluted sample on Baird Parker Agar (Ultimed; Castellar del Vallès, Spain) and incubating at 37 ± 1°C for 24 h.
Application of the essential oils
Evaluation of essential oil antimicrobial activity: Addition technic was used to determine the antimicrobial activities of essential oil according to the technic described by Dègnon et al. [17]. Volumes of 0.25 mL; 0.5 mL at 1 mL of the essential oil were applied on all the surface of 70 g of smoked fish with a swab to determine the low concentration which could inhibit the microflora on smock fish in 24 h. For each test, a control sample (without essential oil) was prepared. The samples tested and control samples were incubated during 72 h at 30°C for Total Aerobic Mesophilic Flora, 72 h at 25°C for yeasts and moulds; 30°C during 48 h for Total Coliforms and 48 h at 37°C for Staphylococcus aureus. Also, each concentration (0.25 mL/70g; 0.5 mL/70 g and 1 mL/70 g) have been tested for 24 h. These tests were done in order to determine the weakest oil concentration being able to neutralize the microflora and conserve smoked fish for one week.
Data processing
The results were analyzed by the variance method (ANOVA) using the STATISTICA software version 6.0 (treatment by ANOVA 1 factor). Comparison of the means was performed by the Tukey's test at 5%.
Results and Discussion
As shown the Figure 2, result of microbial analysis of smoked fish indicated that samples are contaminated by Total Aerobic Mesophilic Flora (TAMF) (4.3 log10 CFU/g), Total Coliform (TC), fungi (yeasts and moulds) and Staphylococcus aureus. But we note an absence of Staphylococcus aureus, yeasts and moulds in smoked fish just after smoking. The level of smoked fish contamination varied according to process of fish treatment. The results corroborate those of Dègnon et al. [17]. We observe that smoked fish took on selling sites was most contaminated in micro-organisms than those took just after smoking. This difference could be explained by the smoking temperature and smoke effect [18]. Maybe this happened because smoking can keep the fish uncontaminated for a special period of time. The temperature of hot smoking fish varies from 60 to 80°C. This evolution of temperature would contribute strongly to reduce level of microbial contamination. The highest contamination of smoked fish in selling could be explained by post-harvest handling, processing and marketing [19]. The exposure of fish smoked to ambient air, the contact of fish with the hands of the vendor and/or the hands of the customer contributes also to smoke fish contamination. This contamination could also result from post process recontamination due to cross-contamination by dirty equipment or unhygienic environment. Indeed, the majority of people of smoked fish manufacturing and marketing do not observe elementary rules of hygiene. It was shown by the highest presence of Total Aerobic Mesophilic Flora (TAMF) in smoked fish as noticed by Djinou [5] and Oulaï et al. [4]. Dione [20] and Dègnon et al. [17] noted a high presence of Total Aerobic Mesophilic Flora (TAMF) in smoked fish. The high fungi contamination of smoked fish was already reported by Thiam and Ducommun and Oulaï et al. [21,4]. Total Coliforms are human and animal digestive tract hosts. Their presence is due to a fecal contamination [22]. The workshops of manuring and even the markets do not have a device for hands washing and disinfection. Thus, the requirement to wash the hands before each activity is not observed on smoking and selling sites.
Staphylococcus aureus was not into samples taken on smoking sites. This result is in conformity with that of Dègnon et al. [17]. Staphylococcus aureus are massively present in smoked fish taken on selling sites. This high presence of Staphylococcus aureus in smoked fish on selling sites is justified by recontamination. Indeed, according to Dègnon et al. [17], the high contamination of these samples would result from the low level of hygiene. Studies of these authors carried out on shrimps showed that the exposure of the smoked product for selling could also constitute a source of contamination (Figure 2).
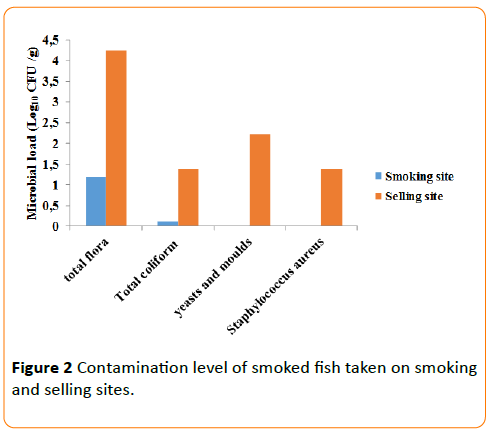
Figure 2: Contamination level of smoked fish taken on smoking and selling sites.
Effect of essential oil concentrations are shown in Table 1. According to this table, concentrations of 0.5 and 1 mL per 70 g of essential oil inhibit completely microbial development on smoked fish during 24 hours. However, for 0.25 mL per 70 g L. multiflora inhibits completely Staphylococcus aureus and coliform development during 24 hours but not Total flora, Yeasts and Moulds which are inhibited respectively with 59.87% and 63.92% at the same time. This capacity of essential oil to inhibit the microbial development in smoked fish is explained by its strong content of antimicrobial composed [23,24].
| Essential oil concentrations (mL/70g of smoked fish) |
Microbial load in smoked fish (Log10 CFU /g) in 24 h |
| Microbial Population and RR(%) |
Total flora |
Total Coliform |
Yeasts and Moulds |
Staphylococcus aureus |
| control |
3.29 ± 1.12 |
2.30 ± 0.98 |
2.19 ± 0.21 |
2.38 ± 0.92 |
| 0.25 |
Test |
1.32 ± 0.36 |
?1 |
0.79 ± 0.42 |
?1 |
| Reducing (%) |
59.87a |
100a |
63.92a |
100a |
| 0.5 |
Test |
?1 |
?1 |
?1 |
?1 |
| Reducing (%) |
100b |
100a |
100b |
100a |
| 1 |
Test |
?1 |
?1 |
?1 |
?1 |
| Reducing(%) |
100b |
100a |
100b |
100a |
Table 1: Effect of application of L. multiflora essential oil on the contamination level of mackerel in 24 h.
The behaviors of each germ during the conservation period after application of the essential oil have been seen in Figures 3-6. The results show that until the first three day, any growth of germs has been noted in smoked fish. But, there were growth with a high number of germs on the control smoked fish samples (without EO), that means deterioration of smoked fish. Studies of Dègnon et al. [17] confirm these results.
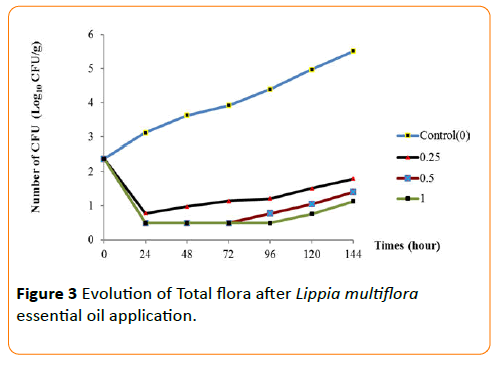
Figure 3: Evolution of Total flora after Lippia multiflora essential oil application.
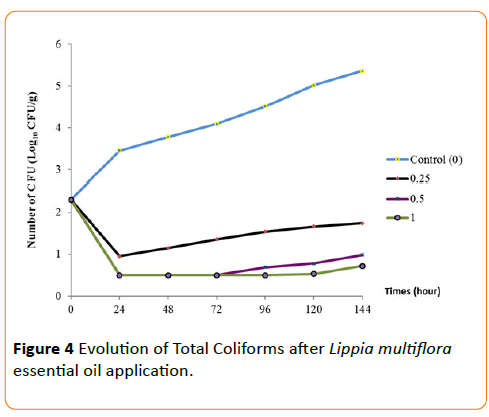
Figure 4: Evolution of Total Coliforms after Lippia multiflora essential oil application.
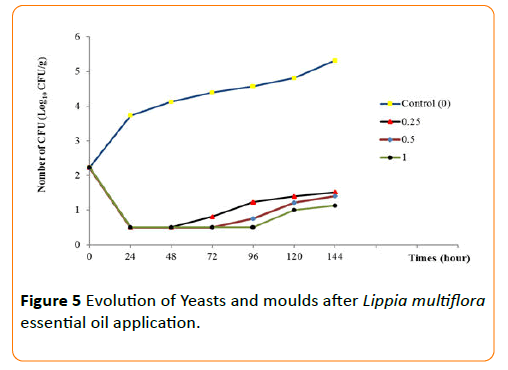
Figure 5: Evolution of Yeasts and moulds after Lippia multiflora essential oil application.
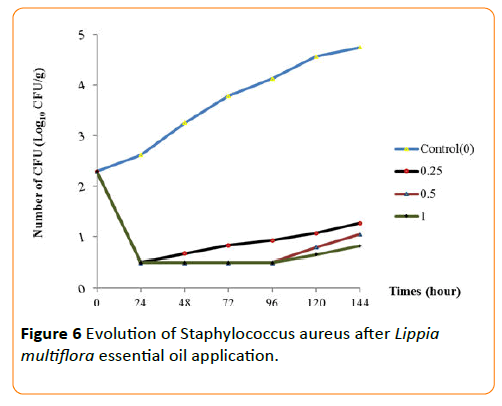
Figure 6: Evolution of Staphylococcus aureus after Lippia multiflora essential oil application.
However, only concentration of 1 mL/70 g inhibited microbial growth. With 0.5 mL/70 g, the microbial growth is inhibited during the first three days during smoked fish conservation. This ability of essential oil to permit the preservation of smoked fish is explained by a high content of bio-actives components. This antimicrobial activity of essential oil of L. multiflora was already noted by Okpekon et al. and Goly et al. [25,24].
After this period, bio-protection of smoked fish leads by essential oil decrease progressively. This reduction in activity could be due to the remanence effect of essential oils because, they are very volatile substances. Their application and their exposure to ambient air involve a progressive disappearing of the volatile molecules of essential oil.
Conclusion
The present study revealed that there is a potential risk of recontamination of smoked fish, which is relation to the low level of hygiene applied in the production of that food. In vitro antimicrobial tests indicated that essential oil of L. multiflora has a pronounced antimicrobial activity. In order to seek natural antimicrobic products capable to extend preservation of smoked fish, this study has shown that L. multiflora essential oil could be used. Tests used to preserve smoked fish by addition of essential oil made it possible to prolong in a substantial way the shelf life of the product. At the fourth day of conservation, the fish is an acceptable microbiological quality. The results obtained show that the preservation of smoked fish by incorporation of essential oil increased the shelf life of the product without chemical conservative addition. However, this protection is not for a long time due to the volatile property of the essential oil.
Conflict of Interest
All authors have contributed equally to the realization of this work. None of the contributing authors has any conflict of interests relevant to the subject matter or materials discussed in the manuscript. No funding or other financial support was received.
18267
References
- FAO (2000) United Nations Food and Agriculture Organization, FAO yearbook, Fishery statistics capture production86: 99-100.
- Diop MB, Destain J, Tine E, Thonart P (2010)Fish preservation in Senegal: potential use of lactic acid bacteria and their antibacterial metabolites. Biotechnologies AgronomieSociété et Environnement14: 341-350.
- Itoua O (1989) Production and consumption of smoked (Mokalu) fish in Congo: technical, hygienic and socio-economic aspects. Thesis of doctorate in Veterinary medicine of the School Inter States of sciences of Sheik Anta Diop University of Dakar, p:170.
- Oulaï FS, Koffi AR, Koussemon M, Dje M, Kakou C, et al. (2007) Evaluation of the microbiological quality of the traditionally smoked fish Ehtmalosafimbriata and Sardinellaaurita. Microbiol Food hyg 19: 37-42.
- Djinou HPAB (2001) Study of the microbiological quality of traditionally smoked fish in Côte.d'ivoire and intended for export. Thesis of Doctorate in Veterinary medicine, Dakar, p:231.
- Gallot S, Fremy JM (2006) Evaluation of the risks related to the presence of the mycotoxins in the human and animal food chains. Synthetic report/ratio AFSSA, Paris, p:25.
- Goueu B (2006) Contribution to the study of evolution of microbiological quality of smoked fish in Côte.d'ivoire and intended for export. Thesis of doctorate in Veterinary medicine of the School Inter-States of Sciences from Sheik Anta Diop University of Dakar p:137.
- OMS (2003) Healthiness of Food and Health: Analyze situation and prospects p:19.
- Barro N, Ousmane O, Bello AR, Philippe AN, André JI, et al. (2007) Impact of the temperature of sale on the deterioration of the microbiological quality of some street food in Ouagadougou (Burkina Faso). Sciences Newspaper 7: 25-32.
- Demirci F, Guven K, Demirci B, Dadandi Y, Baser KHC (2008) Antibacterial activity of two Phlom is essential oils against food pathogens. Food Control 19: 1159- 1164.
- Rota RM, Herrera A, Martinez RM, Sotomayor JA, Jordan MJ (2008) Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 19:681-687.
- Abena AA, Bioka D, Badila Samba C, Hondi-Assah T, Djatewa M (1997) Tranquillizer and analgesic property of Lippiamultiflora. Pharm MédTradAfr 9: 94-107.
- Simard S, Hachey JM, Colin GJ (1988). The variation of the essential oil composition with the extraction process, the case of Thuyaoccidentalis L and Abiesbalsamea L. J MillWood Techn 8: 561-573.
- De Billerbeck VG, Roques CG, Bessière JM, Fonvieille JL, Dargent R (2001) Effect of Cymbopogonnardus (L) W. Watson essential oil on the growth and morphogenesis of Aspergillusniger. Can J Microbiol 47: 9-17.
- Dègnon GR, Agossou V, Adjou SE, Dahouenou-Ahoussi E, Soumanou MM, et al. (2013) Evaluation of microbiological quality of chinchard (Trachurustrachurus) fish during the process of traditional manuring. Newspaper of Applied Biosciences 67: 5210-5218.
- CTA (1990) To preserve and process fish.Guide technical and methodological. Wageningen, CTA, p:295.
- Koffi-Nevry R, Koussémon M (2012) Microbiological Composition, Processing and Consumer’s Characteristics of Adjuevan, a Traditional Ivorian Fermented Fish. Tropicultura 30: 9-14.
- Dione D (2003). Study of microbiological and chemical quality of braise-dried fish. Memory DEA, livest Prod, Dakar (EISMV) p:30.
- Thiam B, Ducommun G (1993) Natural protection of the plants in Africa. Dakar: ENDA, the Third World p: 213.
- Sami M, Nasri A, Bagheri M, Sharifi H (2013) Microbiological and chemical qualities of cream-filled pastries sold in Kerman city confectioneries, southeast of Iran. Eurasian J Vet Sci 29: 138-142.
- Tatsadjieu LN, Ngang JJE, Ngassoum MB, Etoa FX (2003) Antibacterial and antifungal activity of Xylopiaaethiopica, Monodoramyristica, Zanthoxylumxanthoxyloïdes and Zanthoxylumleprieuri from Cameroon. Fitoterapia 74: 469-472.
- Goly C, Soro Y, Kassi B, Dadie A, Soro S, et al. (2015) Antifungal activities of the essential oil extracted from the tea of savanna (Lippiamultiflora) in Côte d’Ivoire. Int JBio ChemSci 9: 24-34.
- Okpekon T, Yolou S, Gleye C, Roblot F, Loiseau P, et al. (2004). Antiparasitic activities of medecinal plants used in Ivory Coast. J Ethnopharmacol90: 91-97.











