Ugwu MC1*, Anie CO2,3, Ibezim EC3 and Esimone CO2
1Department of Pharmaceutical Microbiology and Biotechnology, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria
2Faculty of Pharmacy Delta state University, Abraka Delta State, Nigeria
3Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
Corresponding Author:
Dr. Ugwu MC
Department of Pharmaceutical Microbiology and Biotechnology
Nnamdi Azikiwe University, Awka, Anambra State, Nigeria
Tel: +234 8039460570
E-mail: mc.ugwu@unizik.edu.ng
Received Date: September 23, 2014 Accepted Date: February 11, 2016; Published Date: February 25, 2016
Copyright: © 2016 Ugwu MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
MRSA; Nasal; Antibiotics; Nigeria; Resistance
Introduction
Staphylococcus aureus is a species of bacteria commonly found on the skin and or in the noses of healthy people [1]. The nose is one of the few openings that bacteria have direct access to get inside the body. Thus the nose and nasal passages can be perfect environments for some bacteria. S. aureus has been recognized as one of the most important bacteria pathogen significantly contributing to hospital and community-acquired infections all over the world [2]. It is the most frequently isolated pathogen causing bloodstream infections, skin and soft tissue infections, and pneumonia. Diseases caused by S. aureus include folliculitis, boil, furnculosis, scalded skin syndrome, conjunctivitis, paronychia, mastitis, and toxic shock syndrome [3]. Attempts to control these diseases through the use of antimicrobial agents particularly antibiotics have led to increased prevalence of resistance to these agents [4,5]. The S. aureus diseases and their high mortality were greatly reduced with the use of penicillin in the early 1940s [1]. This success was short-lived with the emergence of Penicillin Resistant Staphylococcus aureus (PRSA) producing beta-lactamase [1]. The betalactamase enzyme inactivates the penicillin antibiotic. Methicillin, a beta-lactamase-resistant beta-lactam, provided new treatment options for PRSA infections. However, the emergence of Methicillin-Resistant Staphylococcus aureus (MRSA) that is cross resistant to all beta-lactams thwarted the treatment options for staphylococcal infections. MRSA can colonize healthy people at a lower rate, about 1- 8%, and represents a potent and increasingly prevalent risk factor for subsequent S. aureus infections [6]. MRSA has evolved resistance not only to beta-lactam antibiotics, but also to several classes of antibiotics. MRSA plays crucial role in diseases acquisition from the community and there has been a global increase in the number of infections caused by MRSA [1,7]. However, data concerning the frequency of nasal carriage of MRSA in the Agbor community is not known. Thus this study was designed to determine the prevalence and antibacterial susceptibility profile of MRSA nasal carriage among healthy students of Agbor college of Education. This we hope may influence antibiotic–use decisions/policies.
Methods
Collection and purification of bacteria isolates
Strains of Staphylococcus aureus were isolated from nostrils of 150 male and 150 female students of college of education Agbor, Delta state, Nigeria using sterile swab sticks. The swabs were immediately inoculated on mannitol- salt agar (Oxoid, England) and incubated for 24 h. Colonies which caused fermentation of mannitol were isolated, Gram-stained and examined microscopically. Thereafter, all Gram-positive cocci in clusters were stored in an agar slant at 4°C for further identification/characterization.
Identification of bacterial Isolates
All Gram-positive cocci isolates that were in clusters and that fermented mannitol were subjected to standard biochemical characterization tests for Staphylococcus aureus [8].
Phenotypic detection of MRSA using the oxacillin disc
Susceptibility of all the S. aureus isolates was conducted by means of the agar screening method on Muller-Hinton agar using Oxacillin sensitivity disc. The S. aureus isolates were standardized to 0.5 Mcfarland standards and were inoculated aseptically onto the nutrient agar plates. The plates were incubated for exactly 24 hours at 37°C.
Antibiotic susceptibility testing (AST)
The antibiotic resistance pattern of the isolates was determined against nine antibiotics using Kirby – Bauer disc – diffusion method (1966) following the CLSI (2010) guidelines. Briefly, the isolates were grown in Nutrient broth at 37°C for 24h. Two loopfuls (0.08 ml) of the suspension of each isolate, standardized by matching with 0.5 MCF were inoculated into 20 ml of sterile molten agar in 10 cm diameter Petri dishes and mixed. The plates were allowed to set and the antibiotic sensitivity disc was aseptically placed on their surfaces. The plates were incubated at 37°C for 24 h and the resultant Inhibition Zone Diameters (IZDs) measured and recorded. These were then interpreted as susceptible, intermediate and resistant according to standard specifications of CLSI.
Results and Discussion
Nasal carriage of S. aureus represents a potent and increasingly prevalent risk factor for subsequent S. aureus infection as strains of Community-Associated (CA)-MRSA that cause infections in healthy people have been detected [9]. In Agbor Delta state, no data exist concerning nasal carriage of MRSA. Thus it is important to determine the incidence of S. aureus and MRSA nasal carriage as this can influence antibiotic-therapy decisions. Out of three hundred nasal swab samples collected and screened, a total 218 (72.7%) of the isolates were found to be S. aureus based on morphology and biochemical tests. The prevalence rate from female to male individuals were 103 (68.6%) and 115 (76.6) respectively as shown in table 1. There was no observed significant difference in colonization rate of S. aureus between the male and female group (P0.05). This observation is line with the findings of Ajoke et al. [1] in Jos, North-central Nigeria and Okwu et al. [10] in Okada, South west Nigeria. They reported that sex is not a remarkable determinant in S. aureus colonization.
| Source |
Number sampled |
Number of samples collected |
Number |
percentage (%) |
| Male |
150 |
150 |
103 |
68.6 |
| Female |
150 |
150 |
115 |
76.6 |
| TOTAL |
300 |
300 |
218 |
72.7 |
Table 1: Frequency of isolation of S. aureus from healthy community individuals in college of Education Agbor
Table 2 shows the prevalence rate of MRSA colonization among healthy individuals in community of the S. aureus isolated was 56 (18.7%). Colonization has been recognized as an important step in the chain of events that leads to S. aureus infections. Individuals are first colonized, invaded and infected. Thus, colonization with S. aureus is a major risk factor for staphylococcal infections [11-13]. The colonization rate (18.7%) recorded in our study is different from the reports of Onanuga et al. in a study conducted in Zaria, Northern Nigeria. They recorded a higher rate of MRSA Colonization (69%) among healthy women. MRSA was detected using oxacillin (30 μg) disc which has high efficiency to detect MRSA as an alternative to PCR in resource constrain areas [1,10].
| Source |
MRSA Number |
Percentage (%) |
| Male |
30 |
53.6 |
| Female |
26 |
46.4 |
| TOTAL |
56 |
100% |
Table 2: Prevalence of MRSA among the healthy students
From Figure 1 the antibiotic resistant pattern of the MRSA isolates was: Amoxicillin 30 (54%) > Streptomycin 25 (45%) > Amoxicillin-clavulanic acid 14 (25%), >Erythromycin 13 (23%) >Chloramphenicol 12 (21%),> Co-trimoxazole 10 (18%),>Ofloxacin 8 (13%),> Ciprofloxacin9 (16%) > Gentamicin 5 (9%). The MRSA isolates recorded a high resistance to Amoxicillin and Streptomycin among all the tested antibiotics. The highest level of resistence was observed to the amoxillin, a penicillin.
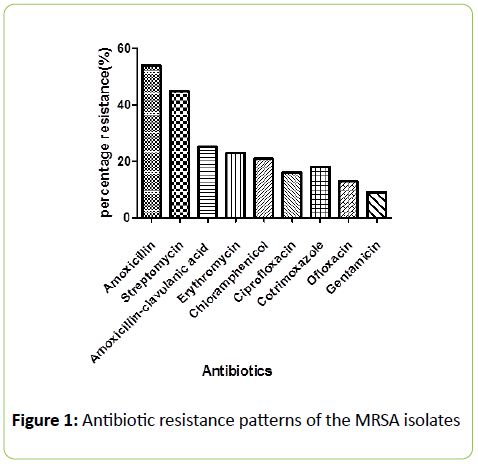
Figure 1: Antibiotic resistance patterns of the MRSA isolates
This is in agreement with the previous reports of Onanuga et al. [14], Rosina and Estifanos, [15], Nkwelang et al. [16] and Rasamiravaka et al. [7]. This observation is related to common prescribing of the penicillin antibiotics [13]. It should however be noted that Amoxicillin-clavulanic acid (Augmentin®) recorded a better effect than amoxicillin against the MRSA isolates. This shows that the resistance of MRSA isolates to amoxicillin is due to β-lactamse inactivation [17]. The clavuanic acid present in the Amoxicilin –clavuanic acid combination (Augmentin®) offers protection to the β-lactam chemical ring nucleus present in the amoxicillin moiety to enhance the activity of Amoxicillin. Several studies have reported that in S. aureus there are β-lactamase enzymes-producing strains [18-21]. In this study most of the isolates were susceptible to Gentamicin (96%) Ofloxacin (86%) and Ciprofloxacin (84%).The sensitivity profile of the MRSA isolates to the flouroquinolones and gentamicin is in line with a previously published work [1]. The high susceptibility was attributed to absence of genes conferring resistance among the isolates (Figure 2). The high sensitivity to the non-beta-lactam antibiotics supports the recommendation of Onanuga that non-beta-lactam antibiotics are preferred drugs for the treatment of Community-Acquired (CA) MRSA infections [22,23].
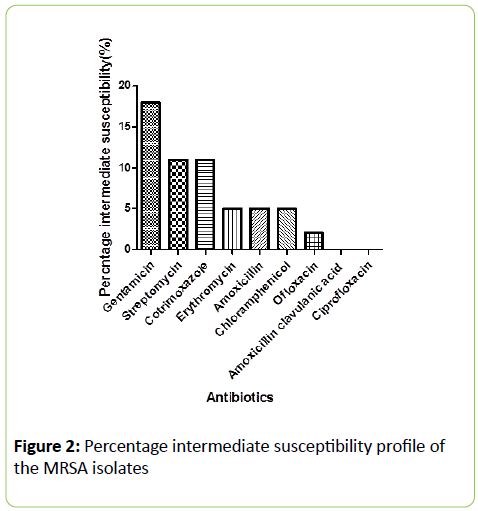
Figure 2: Percentage intermediate susceptibility profile of the MRSA isolates
Conclusion
The study has established prevalence of MRSA among healthy subjects in the institution. The MRSA isolates showed multiple drug resistance to the beta-lactam, Amoxicillin and other commonly prescribed antibiotics: Spectromycin, cotrimoxazole and Erythromycin. Therefore, there is an urgent need to reassess policies on antibiotics use.
8451
References
- Ajoke OI, Okeke IO, Odeyemi OA, Okwor AEJ (2012) Prevalence of methicillin-resistant Staphylococcus aureus from healthy community individuals volunteers in Jos south,Nigeria. Journal of Microbiology, Biotechnology and food scienses 1: 1389-1405.
- Leski T, Oliveira D, Trzcinski K, Sanches IS, Aires de Sousa M, et al. (1998) Clonal distribution of methicillin-resistant Staphylococcus aureus in Poland. Clin Microbiol 36: 3532-3539.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45: 493-496.
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520-532.
- Lowy FD (2003) Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 111: 1265-1273.
- Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, et al. (2004) Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364: 703-705.
- Rasamiravaka T, Rasoanandrasana S, Zafindraibe NJ, Rakoto Alson AO, Rasamindrakotroka A (2013) Evaluation of methicillin-resistant Staphylococcus aureus nasal carriage in Malagasy patients. J Infect Dev Ctries 7: 318-322.
- Cheesbrough M (2006) Biochemical tests to identify bacteria. District Laboratory practices in tropical Countries part 2: 62-70.
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, et al. (2003) Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine Leukocidin genes: Worldwide emergence. In Emerging Infectious Disease 9: 978-984.
- Okwu M, Bamgbala S, Aborisade W (2012) Prevalence of Nasal Carriage of Community-associated Methicillinresistant Staphylococcus aureus(CA-MRSA) among Healthy Primary School Children in Okada, Nigeria. Journal of Natural Sciences Research 2: 61-67.
- Ako-Nai AK, Ogunniyi AD, Lamikanra A, Torimiro SE (1991) The characterisation of clinical isolates of Staphylococcus aureus in Ile-Ife, Nigeria. Microbiol 34: 109-112.
- Holtfreter S, Grumann D, Schmudde M, Nguyen HTT, Eichler P, et al. (2007) Clonal Distribution of Superantigen Genes in Clinical Staphylococcus aureus Isolates. Journal of clinical microbiology 45: 2669-2680.
- Ugwu MC, Mokwe N, Ejikeugwu PC, Gugu TH, Enemor EC, et al. (2015) Antibiogram of Staphylococcus Aureus from Healthy School Pupils in Agulu, Southeastern Nigeria. International Journal of Research in Pharmacy and Biosciences 2: 5-9.
- Onanuga A, Oyi AR, Onaolapo AJ (2005) Prevalence and susceptibility pattern of methicillin resistant Staphylococcus aureus isolates among healthy women in Zaria, Nigeria. African Journal of Biotechnology 4: 1321-1324.
- Rosina G, Estifanos K (2007) Nasal carriage and drug sensitivity of Staphylococcus aureus among healthy Workers of Jimma University Specialized Hospital, Southwestern Ethiopia. Ethiopian Journal of Health Sciences 17: 224-228.
- Nkwelang G, Akoachere JTK, Kamga lH, Nfoncham ED, Ndip RN (2009) Staphylococcus aureus isolates from clinical and environmental samples in a semi-rural area of Cameroon: Phenotypic characterization and Statistical analysis of isolates. African Journal of Microbiology Research 3: 731-736.
- Ugwu MC, Odimegwu DC, Ibezim EC, Esimone CO (2009) Antibiotic resistance patterns of Staphylococcus aureus isolated from nostrils of healthy human subjects in a southeastern Nigerian locality. Maced J of Med Sci 12: 294-300.
- Cowan SI, Steel KJ (1993) Cowan and Steel’s Manual for the identification of medical bacteria. J Clin Pathol 46: 975.
- Emmerson M (1994) Nosocomial staphylococcal outbreaks. Scand J Infect Dis Suppl 93: 47-54.
- Clapper WE, Meade GH (1963) Normal Flora of the Nose, Throat, and Lower Intestine of Dogs. J Bacteriol 6: 327-335.
- Wertheim HFL, Melles DC, Vos MC, Leeuwen WV, Belkum AV, et al. (2005) The role of nasal carriage in Staphylococcus aureus infections. The Lancet 5: 751-762.







