Background: Staphylococcus aureus is a known cause of an array of infections ranging from minor skin and soft tissue infections to chronic bone infections and overwhelming septicemia and endocarditis. Methicillin resistant Staphylococcus aureus (MRSA) is problematic, as the therapeutic outcome of MRSA infections is much worse than those caused by methicillin sensitive strains.
Aim: This study was conducted with an aim to determine the prevalence of MRSA among clinical isolates of Staphylococcus aureus and also determine the antimicrobial susceptibility pattern of MRSA isolates.
Methods: This cross sectional study was carried out from November 2011 to October 2013 in which 510 non-repetitive clinical isolates of S. aureus were identified by conventional phenotypic methods. MRSA isolates were identified using cefoxitin (30 μg) disc diffusion method. Complete antimicrobial susceptibility profile was also determined by Kirby Bauer disc diffusion method. Susceptibility testing to vancomycin was done by E-test.
Results: The prevalence of MRSA among S. aureus isolates was found to be 20.2%. None of the isolates were sensitive to penicillin. However, all the isolates were found to be sensitive to vancomycin, teicoplanin and linezolid. Varying levels of resistance was seen to other antibiotics.
Conclusion: In this study, the prevalence of MRSA isolates was 20.2% which is much less as compared to other studies both in India as well as Western world. This study recommends routine screening of MRSA by cefoxitin disc and also determining the complete antimicrobial susceptibility profile of these isolates to treat these infections effectively.
Keywords
Methicillin resistant Staphylococcus aureus (MRSA); Cefoxitin; E-test; Clinical and Laboratory Standards Institute (CLSI)
Introduction
Staphylococcus aureus has been renowned as an important cause of human disease for more than 100 years [1]. Alexander Ogston first isolated Staphylococcus aureus from a surgical abscess in 1880 and described the role of S. aureus in localized infection and septicemia, including the use of animal models for infection [2,3]. Staphylococcus aureus is recognized as a cause of a wide range of infections, ranging from minor skin infections and chronic bone infections to devastating septicemia and endocarditis [4]. Significant events in the evolution of S. aureus have included the development of methicillin resistance, now a problem for many hospitals around the world. The recent emergence of community strains of S. aureus that are not only methicillin resistant but also harbor genes associated with increased virulence has become a therapeutic challenge [5,6]. Methicillin-resistant Staphylococcus aureus (MRSA) alone (which probably accounts for less than one-third of all S. aureus infections) caused more deaths in the United States in 2005 than human immunodeficiency virus infection. It also caused more invasive infections than other important bacterial pathogens such as Streptococcus pneumoniae, Haemophilus influenza and Neisseria meningitides [7,8].
MRSA is now endemic in India. Its incidence varies from 25% in Western India to 50% in South India [9,10]. Early detection of MRSA and its susceptibility profile becomes important from treatment point of view because it leaves us with very few treatment options such as glycopeptides and linezolid.
This study was conducted with an aim to determine the prevalence and antimicrobial susceptibility pattern of MRSA isolates at a tertiary care centre in order to implement effective control measures and control their spread.
Materials and Methods
This cross sectional study was carried out from November 2011 to October 2013. A total of 510 non-repetitive clinical isolates of Staphylococcus aureus were isolated from various clinical specimens received in microbiology laboratory of a tertiary care centre. These isolates were identified by conventional phenotypic methods such as colony morphology, Gram’s stain, catalase test, slide and tube coagulase test, growth on mannitol salt agar, DNase production, gelatinase production and phosphatase production.
Identification of MRSA
All the isolates of Staphylococcus aureus isolates were subjected to Cefoxitin disc diffusion testing using a 30 μg cefoxitin disc. The results were interpreted according to CLSI guidelines 2013. An inhibition zone diameter of ≤ 21 mm was reported as methicillin resistant and ≥ 22 mm was reported as methicillin sensitive.
Antibiotic susceptibility testing
All MRSA isolates were tested for their susceptibility to various antibiotics by Kirby Bauer disc diffusion method (Table 1). Since Kirby Bauer disc diffusion method is not recommended for susceptibility testing of Staphylococcus aureus to vancomycin, determination of minimum inhibitory concentration (MIC) has to be done either by E-test or agar dilution method. In this study, we used vancomycin E-strips (bio-Merieux) to determine the MIC of vancomycin (Figure 1). MIC ≤ 2 μg/ml was interpreted as sensitive according to CLSI guidelines.
| S. no. |
Antibiotic |
Concentration |
| 1. |
Penicillin |
10 units |
| 2. |
Erythromycin |
15 μg |
| 3. |
Clindamycin |
2 μg |
| 4. |
Co-trimoxazole |
1.25/23.75 μg |
| 5. |
Ciprofloxacin |
5 μg |
| 6. |
Levofloxacin |
5 μg |
| 7. |
Amikacin |
30 μg |
| 8. |
Teicoplanin |
30 μg |
| 9. |
Linezolid |
30 μg |
Table 1: Antibiotics tested against MRSA isolates by Kirby Bauer disc diffusion method.

Figure 1: E-test for determination of MIC of vancomycin against Staphylococcus aureus strain.
Results
Out of 510 isolates of Staphylococcus aureus, 103 isolates were found to be methicillin-resistant Staphylococcus aureus (MRSA). The prevalence of MRSA was 20.2%.
The various clinical samples from which MRSA were isolated are shown in Figure 2. The most common clinical samples were pus (70) followed by blood (12), central line tip (06) and urine (04).
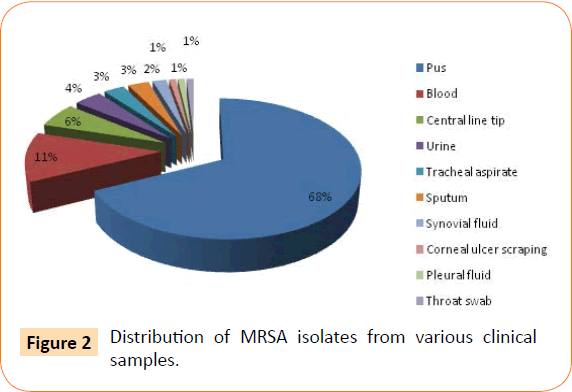
Figure 2: Distribution of MRSA isolates from various clinical samples.
The age of the patients from whom MRSA were obtained ranged from 1.5 months to 79 years of age. It was noticed that amongst the 103 isolates, 69 were from male patients and 34 were from female patients. Male to female ratio was 2:1. Maximum numbers of isolates were from age group 21 to 30 years comprising 35% of the total followed by age group 31 to 40 years (23.3%). Table 2 shows the age and sex wise breakup of the various isolates of MRSA.
| Age |
Male |
Female |
Total |
%age |
| < 10 yrs |
03 |
02 |
05 |
4.8 |
| 11-20 yrs |
05 |
02 |
07 |
6.9 |
| 21-30 yrs |
24 |
12 |
36 |
35 |
| 31-40 yrs |
16 |
08 |
24 |
23.3 |
| 41-50 yrs |
11 |
05 |
16 |
15.5 |
| 51-60 yrs |
04 |
02 |
06 |
5.8 |
| > 60 yrs |
06 |
03 |
09 |
8.7 |
| Total |
69 |
34 |
103 |
100 |
Table 2: Age and sex wise distribution of patients from whom MRSA were isolated.
Antimicrobial susceptibility testing of all MRSA isolates was done by Kirby Bauer disc diffusion method. Antibiogram of the isolates is shown in Figure 3. All the 103 MRSA isolates were found to be resistant to penicillin. In contrast, all the isolates were found to be sensitive to both teicoplanin as well as linezolid. The sensitivity to erythromycin, clindamycin, ciprofloxacin, levofloxacin, cotrimoxazole and amikacin was found to be 24%, 37%, 20%, 27%, 37% and 44% respectively.
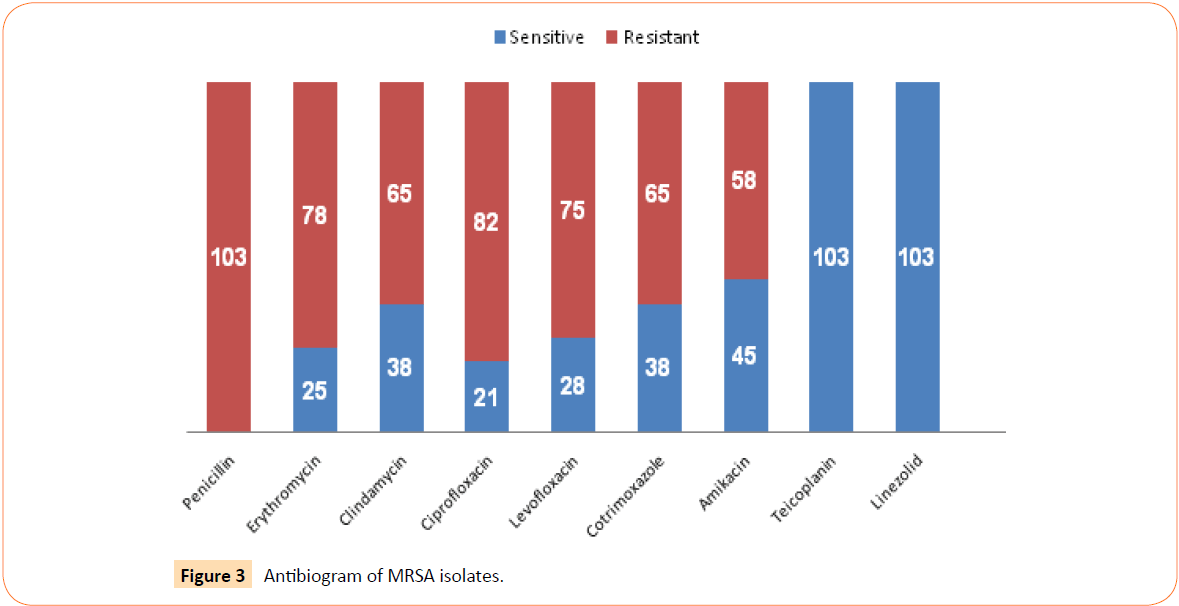
Figure 3: Antibiogram of MRSA isolates.
MIC of vancomycin was determined by E-test and all the isolates of MRSA were found to be sensitive to vancomycin.
Discussion
Staphylococcus aureus has long been recognized as a major human pathogen responsible for a wide range of infections, ranging from mild skin infections to wound infections and bacteremia. Although the introduction of antibiotics over the last 50 years has reduced the mortality rate from these infections, the bacteria have developed resistance mechanisms to all the available antimicrobial agents.
The introduction of penicillin offered an opportunity to successfully treat serious staphylococcal infections. However, in 1944, penicillinase (β-lactamase) producing S. aureus was described which were resistant to penicillin. This enzyme was responsible for the clinical failures that appeared soon after the introduction of penicillin [12]. During the early 1950s, a series of semi-synthetic penicillins were developed that were stable to destruction by bacterial β-lactamases. Methicillin was one such compound which was introduced into clinical practice in 1959. Soon after its introduction, the first methicillin-resistant S. aureus (MRSA) was detected and the first clinical failure of methicillin for the treatment of S. aureus described [11,13]. Methicillin resistance in S. aureus isolates is mediated by an exogenous gene, mecA [12,14]. This gene, mecA encodes for a particular penicillinbinding protein (PBP) called PBP2A, which has a low affinity for methicillin and most other β-lactam drugs which is responsible for the intrinsic resistance of MRSA to almost all β-lactams [15].
Since its discovery, the prevalence of MRSA progressively increased thereafter. One survey of the National Nosocomial Infections Surveillance (NNIS) reported that the hospital prevalence rate of MRSA increased from 2.1% in 1975 to 35% in 1991 [16]. It is now as high up to 60% in certain centres in the United States, but great geographic variations exist worldwide [17]. The prevalence of MRSA in a study from Chennai was reported as 40-50% [18]. Similarly, the overall prevalence reported in Indian network for surveillance of antimicrobial resistance (INSAR) group study was 42% in 2008 and 40% in 2009 [19]. However, in the present study, the prevalence of MRSA was found to be 20.2% which is much less as compared to other studies, both in India as well as Western world.
Though MRSA does not show predilection for any particular age or sex, no age is exempt from these infections. In the present study, it was found that maximum number of isolates came from the age group of 20-40 years, comprising 61.5% of the total isolates (Table 2). Higher incidence of MRSA infections among males has also been reported by Chua T et al. which correlate with our findings [20].
The antimicrobial susceptibility pattern of all the 103 MRSA isolates was determined by Kirby Bauer disc diffusion method and has been shown in Figure 3. It was found that all the isolates were resistant to penicillin. Approximately 75-80% isolates were found to be resistant to ciprofloxacin and levofloxacin which is a cause for concern. About 63% isolates were found to be resistant to cotrimoxazole and the resistance to erythromycin and clindamycin ranged from 63-76%. Similar findings were also observed in the INSAR group study. It was also seen that all the isolates were sensitive to teicoplanin and linezolid.
Disk diffusion sensitivity testing by standard 30 μg vancomycin frequently misclassifies intermediately susceptible isolates as fully susceptible. Presently, MIC determinations by broth or agar dilution or by E test are the gold standard for determining vancomycin susceptibility [21]. In this study, MIC determination by E-test was used to detect vancomycin susceptibility and fortunately 100% of the isolates were found to be vancomycin susceptible.
The degree of morbidity and mortality attributable to MRSA is difficult to assess. Many studies have compared the mortality of MRSA and MSSA bacteremia with conflicting results. A metaanalysis of 31 studies by Cosgrove and colleagues concluded that bacteremia as a result of MRSA is associated with an increased mortality compared with MSSA bacteremia with an odds ratio of 1.93 (95% CI, 1.54 ± 2.42; P<0.01) [11,22].
Vancomycin is considered inferior to β-lactams for the treatment of MSSA bacteremia and endocarditis [19,23]. Therefore, the first-generation cephalosporins are the drugs of choice for the treatment of MSSA infections in patients who are unable to tolerate anti-staphylococcal penicillins. De-escalation of vancomycin to β-lactams should be encouraged in all cases of MSSA. With MRSA isolates being widespread, it is imperative that treating physicians de-escalate to β-lactams once the culture sensitivity results reveal a MSSA isolate. Preservation of glycopeptides and linezolid for use only in MRSA cases should be encouraged [19].
Conclusion
The prevalence of MRSA infection, in the present study, was found to be 20.2%. As the antimicrobial susceptibility profile of MSSA and MRSA differs considerably, the identification of MRSA using cefoxitin disc diffusion testing must be done in all microbiology laboratories. Treatment of multi drug resistant MRSA is problematic because the choice of antibiotics in such cases in very limited. The activity of all available drugs must be tested against the isolate to establish which ones could be used to treat these infections, in order to control the dissemination of such multidrug resistant bacteria.
Conflict of Interest
None identified
6927
References
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520-532.
- Smith G (1982) Ogston'sCoccus: 102 years and still going strong. South Med J 75: 1559-1562.
- Chambers ST (2005) Diagnosis and management of staphylococcal infections of pacemakers and cardiac defibrillators. Intern Med J 35 Suppl 2: S63-71.
- Chambers HF (2001) The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis 7: 178-182.
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. (2003) Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9: 978-984.
- Camargo IL, Gilmore MS (2008) Staphylococcus aureus--probing for host weakness? J Bacteriol 190: 2253-2256.
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, et al. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298: 1763-1771.
- Patel AK, Patel KK, Patel KR, Shah S, Dileep P (2010) Time trends in the epidemiology of microbial infections at a tertiary care center in west India over last 5 years. J Assoc Physicians India 58 Suppl: 37-40.
- Gopalakrishnan R, Sureshkumar D (2010) Changing trends in antimicrobial susceptibility and hospital acquired infections over an 8 year period in a tertiary care hospital in relation to introduction of an infection control programme. J Assoc Physicians India 58: 25-31.
- Hardy KJ, Hawkey PM, Gao F, Oppenheim BA (2004) Methicillin resistant Staphylococcus aureus in the critically ill. Br J Anaesth 92: 121-130.
- Chakraborty SP, Mahapatra SK, Roy S (2011) Biochemical characters and antibiotic susceptibility of Staphylococcus aureus isolates. Asian Pac J Trop Biomed 1: 212-216.
- Hiramatsu K (2001) Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis 1: 147-155.
- Chambers HF, Hartman BJ, Tomasz A (1985) Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest 76: 325-331.
- Panlilio AL, Culver DH, Gaynes RP, Banerjee S, Henderson TS, et al. (1992) Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975-1991. Infect Control HospEpidemiol 13: 582-586.
- Styers D, Sheehan DJ, Hogan P, Sahm DF (2006) Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann ClinMicrobiolAntimicrob 5:2.
- Gopalakrishnan R, Sureshkumar D (2010) Changing trends in antimicrobial susceptibility and hospital acquired infections over an 8 year period in a tertiary care hospital in relation to introduction of an infection control programme. J Assoc Physicians India 58 Suppl: 25-31.
- Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India (2013) Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res 137: 363-369.
- Chua T, Moore CL, Perri MB, Donabedian SM, Masch W, et al. (2008) Molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates in urban Detroit. J ClinMicrobiol 46: 2345-2352.
- Srinivasan A, Dick JD, Perl TM (2002) Vancomycin resistance in staphylococci. ClinMicrobiol Rev 15: 430-438.
- Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, et al. (2003) Comparison of mortality associated with methicillin-resistant and methicillin susceptible Staphylococcus aureusbacteremia: a meta-analysis. Clin Infect Dis 36: 53-59.
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, et al. (2011) Clinical practice guidelines by the infectious disease society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adult and children. Clin Infect Dis 52: e18-e55.








