Keywords
Cytotoxicity; VitaminsB; Amygdalin; HPLC; Chitosanl; Nanoparticles
Introduction
Vitamins are one of the indispensable and vital organic compounds provide no calorie but are important for cellular metabolic reactions. Vitamins are required in small and appropriate amount for normal growth and regulations. Deficit intake of the vitamins leads to the clinical malnutrition and continuous deficiency finally leads to malfunctioning of different enzymes. Some of the vitamin cannot be produced in our cells from metabolic intermediates so are needed continuously for cellular enzymatic regulations [1].
Vitamin B1, thiamin, or thiamine, enables the body to use carbohydrates as energy. It is essential for glucose metabolism, and it plays a key role in nerve, muscle, and heart function [2]. Vitamin B2 or riboflavin is essential for human health. It can be found in grains, plants, and dairy products. It has high importance in breaking down food components, absorbing other nutrients, and maintaining tissues [3]. Vitamin B6 known as pyridoxine is one of the B vitamins that benefit the central nervous system. It is involved in forming myelin. It is not stored by the body, and it is excreted in the urine, so people need to take in Vitamin B6 everyday [4,5]. Folic acid is a form of vitamin B9 that can dissolve in water. It is a key ingredient in the making of the nucleic acid that forms part of all genetic material. Its forms carry out the crucial functions of creating more red blood cells [6].
Vitamin B17 is a naturally occurring chemical compound best known for being falsely promoted as a cancer cure. It is found in many plants, but most notably in the seeds (kernels) of apricots, bitter almonds, apples, peaches, and plums. Amygdalin is classified as a cyanogenic glycoside because each amygdalin molecule includes a nitrile group, which can be released as the toxic cyanide anion by the action of a beta-glucosidase [7].
Our work aimed to determine of some different types of vitamin B and the application of different methods of extracting and analysing them by means of chromatographic analysis then loading them on chitosan (CSNPs) to be ready to study its biological applications on different types of cell lines to assess their cytotoxicity.
Materials
Materials that are used as Vitamin B1, B2, B6 and Vitamin B9 are from USP reference standard. Vitamin B17 is from SIGMA-ALDICH. Fruits as Apple, Apricot, Okra, Almond and Grapes are collected from local markets. All HPLC apparatus are SHIMADZU brand. For nanoparticles Chitosan; ACROS (ORGANICS)- Molecular weight 100,000-300,000 Da ,Different types of Vitamin B (B1,B2,B6,B9,B17), Tripolyphosphate (TPP) and Sodium Tripolyphosphate Pentabasic purchased from SIGMA-ALDRICH - Molecular Weight 367.86 g/mol. For Cytotoxic activity of cell lines as normal human lung fibroblast cell line (WI-38) and a panel of three human cancerous cell lines as Hepatocelluar carcinoma (HePG2), Mammary gland Breast cancer (MCF-7) and Epitheliod Carcinoma Cervix cancer (Hela) [8]. All the five types of cell lines were obtained from ATCC via Holding company for biological products and vaccines (VACSERA), Cairo, Egypt. Doxorubicin was used as a standard anticancer drug for comparison. The reagents RPMI-1640 medium, MTT (3-(4, 5- dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) and DMSO (dimethyl sulfoxide) were purchased from sigma co., St. Louis, USA) and Fetal Bovine serum (GIBCO, UK) was used.
Methods
Vitamins B1, B2, B6 and B9 extraction
Extraction is carried out by collecting fruits that are collected from local markets such as Apple, Apricot, Grapes and Okra that were washed and dried, then 50 g was weighed and cut into small pieces, then extracted with 0.1 NHCl in water bath at suitable temperature and time period approximately 30 minutes and extracts are collected to be treated by HPLC [9].
Vitamin B17 extraction
This process was carried out by collecting Almond, Apple and apricot from local markets that were washed and dried then cutted into small pieces and (3 g of each) were reflux extracted by 100 mL water with 0.1% citric acid for 2.5 h in the condition of a 60°C water bath. The water extract was collected after filtration. The residue was dissolved in 100 mL water with 0.1% citric acid and the previously mentioned process was repeated.
The two times water extract was then combined and diluted to 200 ml. After extraction, the extract was separated from the solid plant material by filtering process. Plant extracts is evaporated to remove the solvent, and then stored in desiccator until it reached the constant mass. Amygdalin is precipitated by adding diethyl ether (10 cm3) to the complex [10].The highest quantity of extracted B17 was obtained from Apricot, so this specimen was later converted in to nanoparticles and then in studying of their cytotoxic activities.
Standard preparation
Vitamin B1 stock standard solution of Thiamine HCl is prepared by dissolving 50 mg of the standard in 0.1 M HCl in 25 mL standard flask, then completed with the acid. The working standard is prepared from the stock standard solution by taking 0.5 mg/mL of the stock (1.0 ml) into 10 mL standard flask and made up to mark with appropriate solvent. The solution was sonicated for 30 min and 20 μL is injected into the column and separated.
Vitamin B2 stock standard solution of Riboflavin is prepared by dissolving 6.9 mg of riboflavin in 100 ml of extraction solution because the extraction solution. The working standard is prepared from the stock standard solution by take 5.1 ml was taken in 10 ml volumetric flask, dissolved then completed with bi-distilled Water. The solution was sonicated for 30 min and 20 μL is injected into the column and separated.
Vitamin B6 stock standard solution of Pyridoxine HCl is prepared by dissolving 20.8 mg of the standard in 25 mL standard flask dissolve then made up to mark with H2O. The working standard is prepared from the stock standard solution by take 2.84 ml in 10 ml volumetric flask complete to volume with H2O .The solution is sonicated for 30 min and 20 μL is injected into the column and separated.
Vitamin B9 stock standard is prepared by dissolving 11.2 mg of folic acid in 25 ml of double distilled water. The working standard is prepared from the stock standard solution by take 5.196 ml was taken in 10 ml volumetric flask. The solution was sonicated for 30 min and 20 μL is injected into the column and separated. Vitamin B17 stock standard is prepared by dissolving 40 mg of amygdalin (vitamin B17) in 25 ml of mobile phase. The solution was sonicated for 30 min and 20 μL is injected into the column and separated [11].
Samples preparation and HPLC conditions
Vitamin B1 the extracts (2.0 g) are weighed into 200 ml standard flask, 30 mL of 0.1 M Hcl is added and the mixture is allow to heat at 40°C for 5 min .The mixture is filtered through ashless filter paper and then centrifuge for 10 min at 1800 rpm. The supernatant is sonicated and 20 μL is injected into the column and separated. Hplc conditions are coulmn bondapack C18 column or equivalent, mobile phase comprised of 90% of 0.01 M sodium mono-hydrogen phosphate and 10% HPLC grade methanol and wave length set at 254 nm. Flow rate 0.60 ml/min. Injection volume 20 μl (Figure 1a and 1b). For Figure 1a and 1b as we examine charts we found that the main peak of VITAMIN B1 in standard solution is compatible with the peak in test solution in retention time at the same wavelength. The mobile phase must be filtered and sonicated for 30 min before use [12,13].
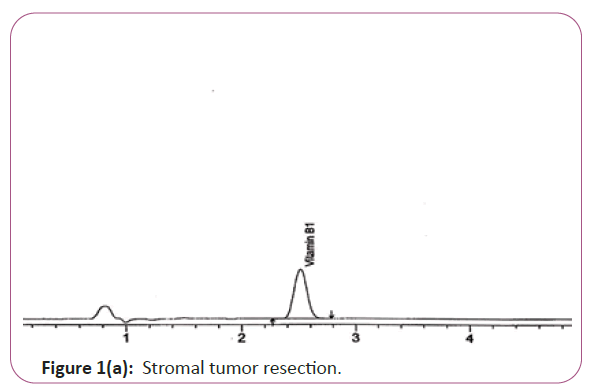
Figure 1a: Stromal tumor resection.
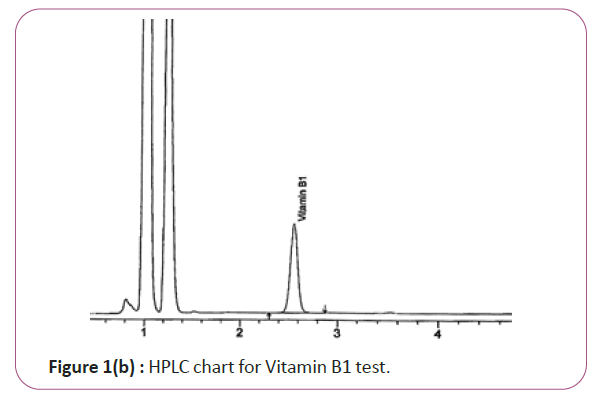
Figure 1b: HPLC chart for Vitamin B1 test.
Vitamins B2, B6 and B9 samples are carried out by weighing 10 gm of extracts then transferred into conical flasks and 25 ml of extraction solution was added. Keep on shaking water bath at 70°C for 40 min. Thereafter, the sample was cooled down filtered and finally the volume was made up to 50 ml with extraction solution. HPLC conditions are column symmetry C18 column (4.6 × 250 mm 5 μ), mobile phase Buffer: methanol (96:4) and wave length set at 280 nm for vitamin B2, 210 nm, for vitamin B6 and B9. Flow rate 1.0 mL/min. Injection volume 20 μl (Figure 2a and 2b). For Figure 2a and 2b as we examine charts we found that the main peak of Vitamin B2 in standard solution is compatible with the peak in test solution in retention time at the same wavelength. The mobile phase must be filtered and sonicated for 30 min before use.
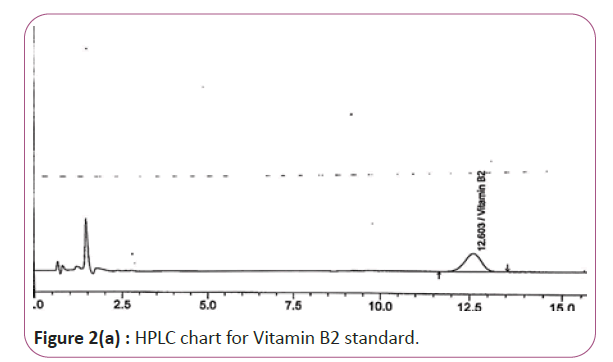
Figure 2a: HPLC chart for Vitamin B2 standard.
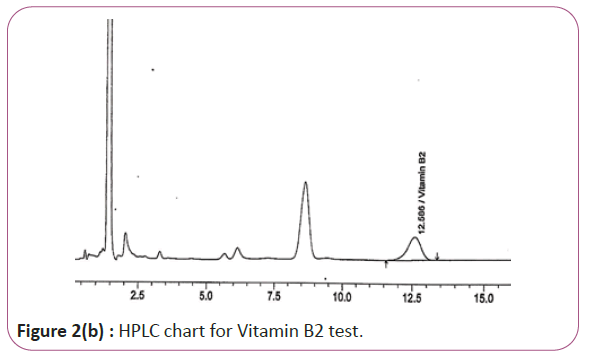
Figure 2b: HPLC chart for Vitamin B2 test.
One gram of dry Vitamin B17 extract was accurately weighed, and then it placed into a 10 cm3 flask along with 10 cm3 of mobile phase (Figure 3a and 3b). For Figure 3a and 3b as we examine charts we found that the main peak of Vitamin B6 in standard solution is compatible with the peak in test solution in retention time at the same wavelength. This sample was sonicated for 5 min. 1 cm3 of this solution was quantitatively transferred into a 10 cm3 volumetric flask. The solution was diluted using mobile phase and mixed and 20 μL of the sample was injected in the HPLC (Figure 4a and 4b). For Figure 4a and 4b as we examine charts we found that the main peak of Vitamin B9 in standard solution is compatible with the peak in test solution in retention time at the same wavelength. HPLC Analysis using mobile phase mix of water and acetonitrile 25:75 (v/v). The flow rate of mobile phase with isocratic elution was 0.9 cm3 min−1. Before analysis, the solutions were filtered through a 0.45 μm millipore filter. The quantification was effected by measuring at 215 nm (Figure 5a and 5b). For Figure 5a and 5b as we examine charts we found that the main peak of Vitamin B17 in standard solution is compatible with the peak in test solution in retention time at the same wavelength. The separation was performed at the temperature of 25°C and using a SUPELCO Analytical HS-C18 Column (4.6 × 250 mm, 5 μm).
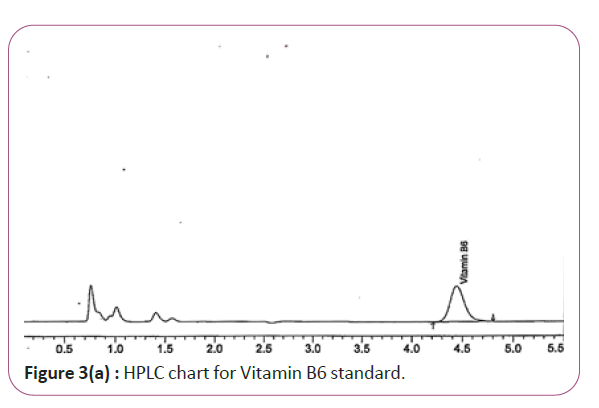
Figure 3a: HPLC chart for Vitamin B6 standard.
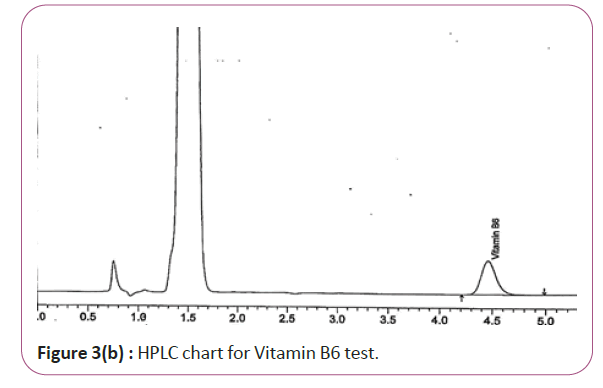
Figure 3b: HPLC chart for Vitamin B6 test.
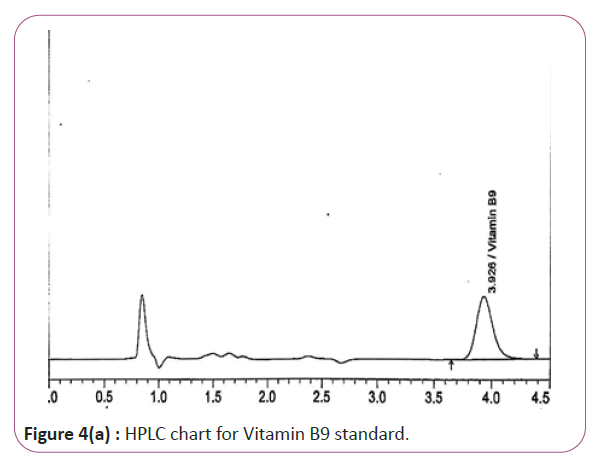
Figure 4a: HPLC chart for Vitamin B9 standard.
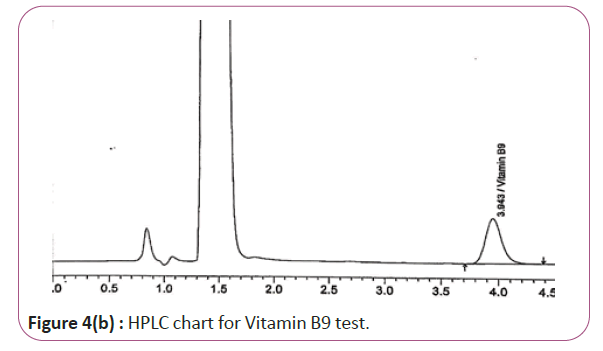
Figure 4b: HPLC chart for Vitamin B9 test.
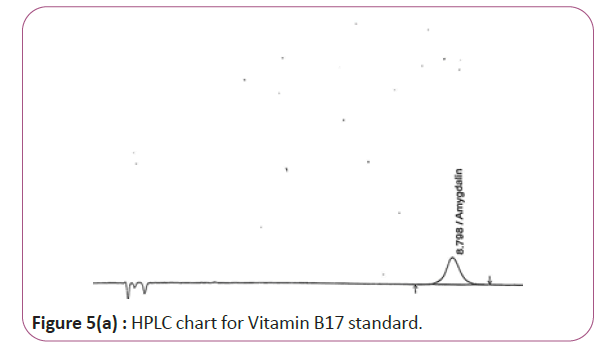
Figure 5a: HPLC chart for Vitamin B17 standard.
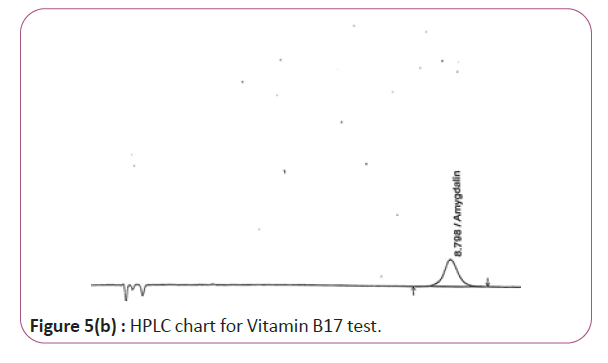
Figure 5b: HPLC chart for Vitamin B17 test.
Loading vitamins with chitosan nanoparticles (CSNPs)
Vitamins in our study will be converted in to nanoparticles with the aid of chitosan to be able to increase viability in our applied applications. Preparation of Chitosan nanoparticles and vitamins B1, B2, B6, B9 and B17 loaded with Chitosan nanoparticles, Chitosan nanoparticles (CSNPs) were prepared by ionic gelation method. CSNPs spontaneously form via the electrostatic attraction between positively charged primary amino groups on Chitosan chains and reversely charged polyanions (from TPP). The resulting opalescent suspension was determined as CSNPs as is illustrated in Figure 6.
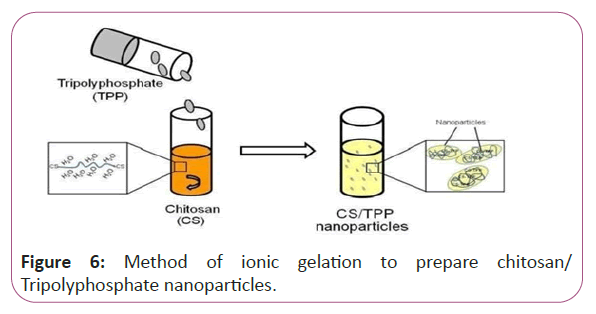
Figure 6: Method of ionic gelation to prepare chitosan/ Tripolyphosphate nanoparticles.
2 mg/ml of Chitosan was dissolved in 1% (w/v) acetic acid and sonicated before the solution became transparent. The addition of TPP solution, at a concentration of 0.7 mg/ml drop wise, to equal volume of CS solution, under continuous stirring at 500 rpm for 30 minutes at room temperature. The resulting opalescent suspension was determined as CSNPs. In addition, Chitosan nanoparticles loaded with vitamin B1, B2, B6, B9 and B17 were prepared by ionic gelation method.
The same procedures were done for preparation of Chitosan nanoparticles loaded with vitamin B1 in the presence of 0.2 gm that was dispersed in 100 ml of CS solution. The resulting opalescent suspension was determined as vitamin B1 loaded with Chitosan nanoparticles. Reaction mixture was centrifuged at 11000 rpm at 4°C for 30 mins using high speed cooling centrifuge. The supernatant was decanted and nanoparticles were suspended in sterile distilled water and recentrifuged at 11000 rpm at 4°C for 30 mins. To separate the nanoparticles, the same procedures were done for preparation of Chitosan nanoparticles loaded with different type of vitamin (B1, B2 ,B6, B9, B17) in the presence of 0.2 gm that was dispersed in 100 ml of CS solution with repeating the same method of vitamin B1 loaded with Chitosan nanoparticles as above.
Vitamins B1, B2, B6, B9 and B17 solution were prepared by solubility of 0.2 gm of Vitamin B1 in 200 ml of disttled water. Scanning electron microscopy (SEM) JEOL-JSM-5400, Japan, SEM operating at 30 kV. Zetasizer measurements Zeta potential (ζ) were determined using a Zetasizer (ZS), (Nano-ZS, Malvern, UK). The measurement was carried out in disposable polystyrene cuvettes at 25°C with measurement angle of 12.8°C and 175°C.
The zetasizer (Nano-ZS, Malvern, UK). Some scan photos are shown below to prove that vitamins are converted in to nano- particles.
Samples that will be used in our applied applications are DOX as a reference drug, vitamins loaded with chitosan symbolized as (CSNPs B9, B6, B2, B17 and B1), chitosan (ch) and chitosan nanoparticles (CSNPs), also vitamins B1, B2, B6, B9 and B17 solutions in normal forms [14].
Vitamins B1, B2, B6, B9 and B17 after loaded with chitosan nanoparticles (CSNPs) are ready to biological application stage (cell line technique).
Cytotoxic activity and MTT Assay
Cytotoxic activity is defined as the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (Bitis arietans) or brown recluse spider (Loxosceles reclusa). The MTT assay is a colorimetric assay for assessing cell metabolic activity. NAD(P)H-dependent cellular oxidoreductase enzymes may, under defined conditions, reflect the number of viable cells present. The inhibitory effects of all the tested vitamins on cell growth using the MTT assay. This colorimetric assay is based on the conversion of the yellow tetrazolium bromide (MTT) to a purple formazan derivative by mitochondrial succinate dehydrogenase in viable cells. Cell lines were cultured in RPMI-1640 medium with 10% fetal bovine serum. Antibiotics added were 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C in a 5% Co2 incubator. The cell lines were seeded in a 96-well plate at a density of 1.0 × 104 cells/ well. The plate was incubated at 37°C for 48 h under 5% Co2. After incubation the cells were treated with different concentration of compounds and incubated for 24 h. After 24 h of drug treatment, 20 μl of MTT solution at 5 mg/ml was added and incubated for 4 h. Dimethyl sulfoxide (DMSO) in volume of 100 μl is added into each well to dissolve the purple formazan formed.
The colorimetric assay is measured and recorded at absorbance of 570 nm using a plate reader (EXL 800, USA). The relative cell viability in percentage was calculated as (A570 of treated samples/A570 of untreated sample) × 100[15].
Statistical analysis
The obtained results were statistically analyzed using GraphPad instat, Version 3.06 (GraphPad) Software Inc., San-Diego,California USA), and the data were expressed as mean ± standard deviations (SD) and it statistically analyzed by one way ANOVA (Analysis of Variance) for multiple comparisons. P values less than 0.05 were considered significant.
Results and Discussion
For HPLC determination of all Vitamins B1, B2, B6, B9 and B17 As we examine HPLC charts (Figures 1a and 1b) (Figures 5a and 5b), we found that every item such as standard solution or test solutions must be injected two injections. For each vitamin at its specified wavelength, we compare peak of standard and peak of test at the same retention time we found that they are approximately the same. By applying the specified calculations we find that the results obtained are close to expected value as shown below.
Vitamin B1 calculations
(Peak area of test/Peak area of standard) × (Weight of standard/25) × (1/10) × (200/Weight taken) × standard activity (99.8%) (Figure 7).
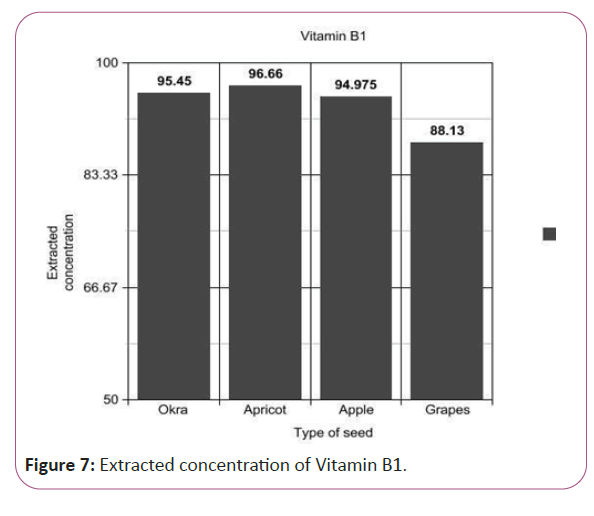
Figure 7: Extracted concentration of Vitamin B1.
Vitamin B2 calculations
(Peak area of test/Peak area of standard) × (Weight of standard/100) × (5.1/10) × (50/Weight taken) × standard activity (99.7%) (Figure 8).
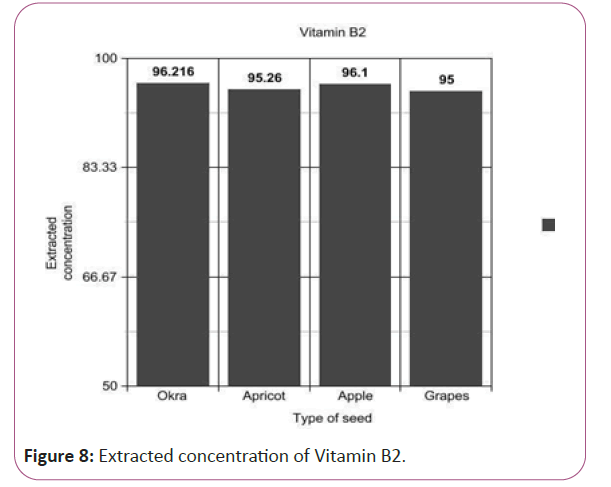
Figure 8: Extracted concentration of Vitamin B2.
Vitamin B6 calculations
(Peak area of test/Peak area of standard) × (Weight of standard/25) × (2.84/10) × (50/Weight taken) × standard activity (99.7%) (Figure 9).
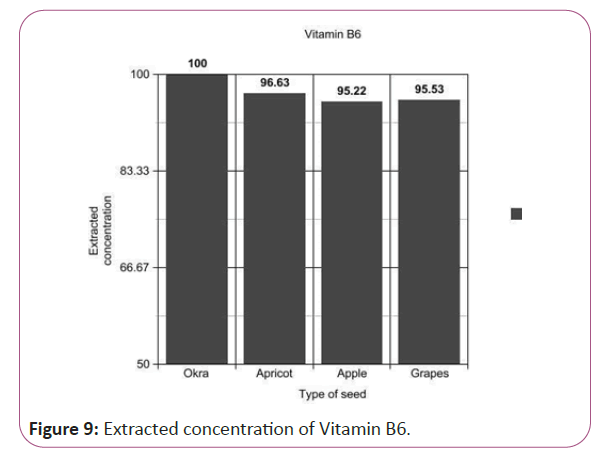
Figure 9: Extracted concentration of Vitamin B6.
Vitamin B9 calculations
(Peak area of test/Peak area of standard) × (Weight of standard/25) × (5.196/10)x(50/Weight taken) × standard activity (98.9%) (Figure 10).
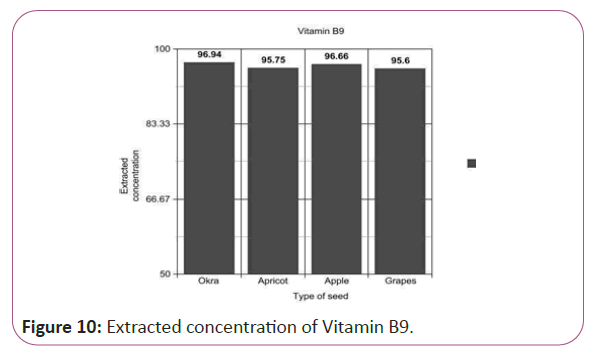
Figure 10: Extracted concentration of Vitamin B9.
Vitamin B17 calculations
(Peak area of test/Peak area of standard) × (Weight of standard/25) × (10/1)x(10/Weight taken) × standard activity(99%) (Figure 11).
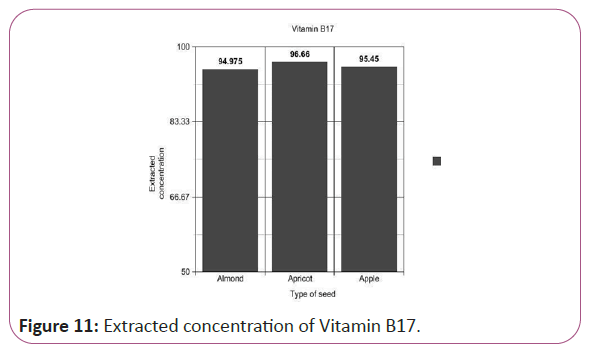
Figure 11: Extracted concentration of Vitamin B17.
Figures 12–16 shows that vitamins B1, B2, B6, B9, and B17 were examined to determine the extent of loading them with chitosan nanoparticles (CSNPs), and the pictures scanned showed the positive extent of the loading process and they were ready to be applied on different cell lines.

Figure 12: Vitamin B1 scan after loaded with chitosan nanoparticles (CSNPs B1).

Figure 13: Vitamin B2 scan after loaded with chitosan nanoparticles (CSNPs B2).

Figure 14: Vitamin B6 scan after loaded with chitosan nanoparticles (CSNPs B6).

Figure 15: Vitamin B9 scan after loaded with chitosan nanoparticles (CSNPs B9).

Figure 16: Vitamin B17 scan after loaded with chitosan nanoparticles (CSNPs B17).
From the obtained results in our study, some chitosan loading vitamins as vitamins CSNPs B6, CSNPs B1 and CSNPs B9 showed excellent results with very low cytotoxic effects on WI38 (normal cell line). It can be safely used in cancer treatment without having any toxic effects on the normal cells. On the other hand, some other chitosan loading vitamin forms showed highly toxic effects on the normal cell line which indicated that it has hazardous effects on normal cell and cannot be safely used as Vitamins CSNPs B17. Vitamin B17 solution (in its normal form) gave highly cytotoxic effects on the tested normal cell line but by chitosan loading it showed greater cytotoxicity (Figures 17-20).
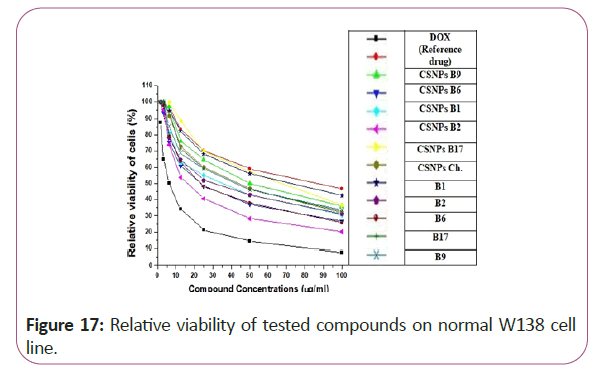
Figure 17: Relative viability of tested compounds on normal W138 cell line.
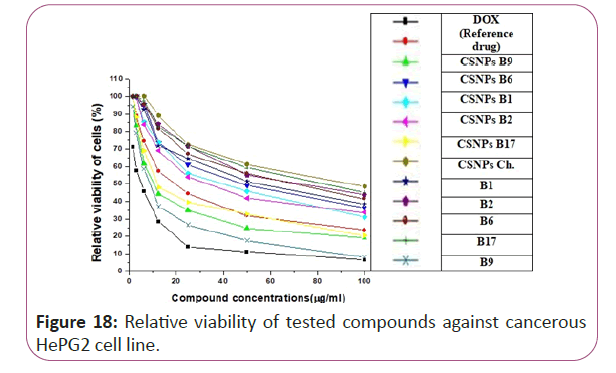
Figure 18: Relative viability of tested compounds against cancerous HePG2 cell line.
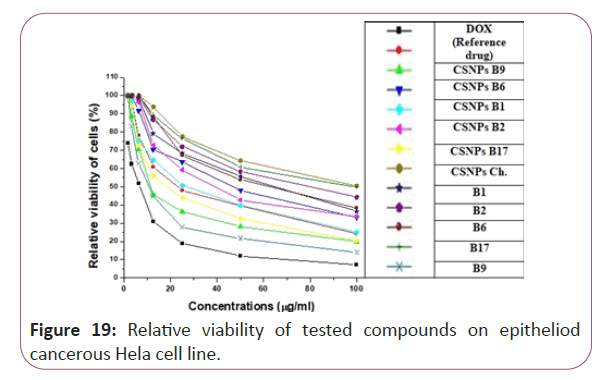
Figure 19: Relative viability of tested compounds on epitheliod cancerous Hela cell line.
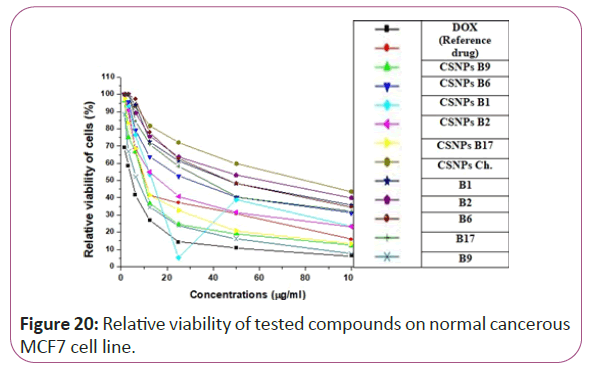
Figure 20: Relative viability of tested compounds on normal cancerous MCF7 cell line.
For different cancerous cell lines, vitamins CSNPs B6 and CSNPs B1 showed excellent results on all tested cell lines with values comparable to the results obtained by doxorubicin (the reference drug). Vitamins CSNPs B9 and CSNPs B17 also showed good results. While, vitamins CSNPs B2, the solution of vitamins B1, B2, B9, B6 and B17 showed relatively weak cytotoxic activities (Table 1).
| Tested compound |
In vitro Cytotoxicity IC50 (µg/ml) |
| WI38 |
HePG2 |
Hela |
MCF7 |
| DOX (Reference drug) |
6.72± 0.5 |
4.50± 0.2 |
5.57± 0.4 |
4.17± 0.2 |
| CSNPs B9 |
76.40± 4.1 |
21.23± 1.9 |
26.78± 1.8 |
14.82± 1.3 |
| CSNPs B6 |
51.36± 3.4 |
13.06± 1.0 |
15.34± 1.2 |
9.91± 0.8 |
| CSNPs B1 |
61.48± 3.6 |
17.15± 1.4 |
20.59± 1.5 |
12.35± 1.0 |
| CSNPs B2 |
33.82± 2.7 |
39.40± 2.4 |
28.17± 2.1 |
24.78± 1.9 |
| CSNPs B17 |
18.73± 1.5 |
36.29 ± 2.3 |
41.32± 2.7 |
19.06± 1.6 |
| CSNPs Ch. |
25.93± 1.9 |
47.21± 2.8 |
45.08± 3.0 |
31.57± 2.4 |
| B1 |
43.07± 3.2 |
83.49± 4.3 |
92.11± 4.8 |
74.13± 3.7 |
| B2 |
65.29± 3.9 |
52.67± 3.2 |
57.63± 3.4 |
46.59± 3.1 |
| B6 |
32.80± 2.5 |
68.16± 3.8 |
71.34± 3.7 |
55.16± 3.6 |
| B17 |
27.14± 2.2 |
63.44 ± 3.6 |
59.15± 3.5 |
48.07± 3.3 |
| B9 |
42.19± 3.3 |
75.03± 3.9 |
86.26± 4.2 |
37.10± 2.8 |
| Ch. |
40.85± 2.9 |
9.48± 0.8 |
11.60± 0.9 |
7.24± 0.5 |
Table 1: Cytotoxic activity of the tested compounds against different cell lines show that Vitamins are highly promising to be used in cancer therapy and antioxidant treatments, respectively.
Inspire of the strong cytotoxic activity of chitosan loading vitamin B17 (CSNPs B17) against cancerous cell lines form, but it also showed also highly cytotoxicity against normal cell lines. Even vitamin-B17 solution showed the same cytotoxic effect on normal cell line. It can be explained by the cyanide poisoning which results from cyanide release. Many recent previous studies documented its clinically ineffective effects in treating cancer [16]. These results completely destroyed the false promotion as a cancer cure. These findings were in contrast to the old facts discovered by Krebs (1940) who documented that inside body Beta-glucosidase enzyme splits vitamin B17 into toxic cyanide ions and benzaldehyde that tend to kill tumor cells in a sort of pseudo-apoptosis. In human body, enzyme Rhodanese is present only in normal tissues with maximum concentration in the liver that breaks hydrogen cyanide and benzaldehyde into thiocyanates and benzoic acid necessarily in nourishment of healthy cells and production of vitamin B12. Krebs also described the mode of action of amygdalin by characterizing the existence of enzyme Rhodanese in whole body except tumor cells. Contrarily, the enzyme beta-glycocidase was found only in cancerous cells. Amygdalin is decomposed by the action of enzyme ß-D- glucosidase found in cancerous cells not healthy cells to yield hydrocyanic acid [17,18]. These conflicting explanations may depend on free released cyanide concentrations.
The excellent cytotoxic activities of both CSNPs B6 and CSNPs B1 can be explained by the ability of pyridoxine to lower the levels of 8-hydroxyguanosine (8-OHdG), 4-hydroxy-2-nonenal (4-HNE, oxidative stress markers) and inducible nitric oxide (NO) synthase protein [19]. It also reduces homocysteine concentrations in blood [20]. So, these results can be considered as promising starting point for the development of new anticancer agents from pyridoxine. Many previous studies documented that thiamine (vitamin B1) reduces cancer cell proliferation mainly by alteration in the regulation of the thiamine-dependent enzyme pyruvate dehydrogenase [21,22].
Conclusion
In conclusion, these results suggest that the chitosan loading nanoparticles of vitamins B6 (CSNPs B6) and B1 (CSNPs B1) can be a novel platform for the successful anticancer drugs.The study showed that fruits are good extraction sources of vitamins B1, B2, B6, B9, and B17. The presence of these vitamins was analyzed and indicated by chromatographic methods in the expected proportions. Chitosan loading nanoparticles of the extracted vitamins were applied against normal and cancerous cell lines. The results showed impressive and starting point for the development of new anticancer agents.8.
36138
References
- B vitamins".Medline Plus, US National Library of Medicine. 28 September 2020.
- KW, Done SH, Gruenberg W (2017) Diseases of the Nervous System - Veterinary Medicine (Eleventh Edition)-hiamine (vitamin B1) is synthesized only in bacteria, fungi, and plants but is an essential nutrient for animals. 14:1155–1370.
- Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR May 2014.
- Choi JH, Yates Z, Veysey M, Heo YR, Lucock M (2014) "Contemporary issues surrounding folic Acid fortification initiatives". Prev Nutr Food Sci. 19 (4): 247–60.
- European Food Safety Authority 27 April 2016). A naturally-occurring compound called amygdalin is present in apricot kernels and converts to hydrogen cyanide after eating. Cyanide poisoning can cause nausea, fever, headaches, insomnia, thirst, lethargy, nervousness, joint and muscle various aches and pains, and falling blood pressure.
- Carrel Alexis and Montrose T. Burrows (1911) "Cultivation of Tissues in Vitro and its Technique". J Exp Med 13 (3): 387–396.
- USP-NF, Dietary Supplements: Water-Soluble Vitamins Capsules/Method 1, The United States Pharmacopeial Convention, Inc., Rockville, MD, USA, 2018.
- Viorica-Mirela G, Socaciu C, Jianu I, Florica R, Florinela F (2006) Identification and quantitative evaluation of amygdalin from apricot, plum and peach oils and kernels. Buletin USAMV- CN. 62:246–253.
- WeiFeng L.V., Ding Y.U., andZheng R (2005) “Isolation andquantitation of amygdalin inapricot kernel and PrunusTomentosa Thunb. by HPLCwith solid phase extraction” J Chromatogr Sci. 43:383-387
- Poongothai S, Ilavarasan R, Karrunakaran CM (2010) Simultaneous and accurate determination of vitamins B1, B6, B12 and alpha-lipoic acid in multivitamin capsule by reverse-phase high performance liquid chromatographic method. Int J Pharm Pharm Sci 2(4):133-139.
- Chen P, Atkinson R, Wolf WR (2009) Single laboratory validation of a high-performance liquid chromatographic-diode array detector-fluorescence detector/mass spectrometric method for simultaneous determination of water-soluble vitamins in multivitamin dietary tablets. J AOAC Int 92(2):680-7.
- Calvo P, Remunan-Lopez C, Villa-Jato JL, Alonso MJ (1997) Novel Hydrophilic Chitosan- Polyethylene Oxide Nanoparticles as protein carrier. J Appl Poly Sci 63: 125-32.
- Y Kawakami, N Nagai, K Ohama, K Zeki, Y Yoshida et al. (2000) Macrophage-colony stimulating factor inhibits the growth of human ovarian cancer cells in vitro Eur J Can 36:1991-1997.
- Milazzo S, Horneber M (April 2015). "Laetrile treatment for cancer". The Cochrane Database of Systematic Reviews 2015(4): CD005476.
- Bromley J, Hughes BG, Leong DC, Buckley NA (2005). Life threatening interaction between complementary medicines: Cyanide toxicity following ingestion of amygdalin and vitamin C. Ann Pharmacother; 39: 1566- 1569.
- Moon JY, Kim SW, Yun GM, Lee HS, Jeon BG (2015) Inhibition of cell growth and down-regulation of telomerase activity by amygdalin in human cancer cell lines. Animal cells and systems, 19(5): 295-304.
- Komatsu S, Yanaka N, Matsubara K, Kato N (2003). Antitumor effect of vitamin B6 and its mechanisms. Biochim Biophys Acta. ; 1647 (1-2):127-130.
- Galluzzi L, Vacchelli E, Michels J, Garcia P, Kepp O et al.(2013). Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene 32: 4995–5004.
- Hanberry BS, Berger R, Zastre JA (2012). High Dose Vitamin B1 Reduces Proliferation in Cancer Cell Lines Analogous to Dichloroacetate. Cancer Chemother Pharmacol 73(3): 585-94.
- In BP Marriott, DF Birt, VA Stallings, AA Yates (2020) Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier) pp. 225–38.






























