Keywords
Ischemic stroke; Secondary hemorrhagic stroke; Fluoxetine; Simvastatin; Infarct volume
Introduction
Only a small percentage of acute ischemic stroke patients reach the hospital in time to be considered for treatment with recombinant tissue plasminogen activator (rtPA),which can minimize permanent brain damage and reduce the risk of longterm disability [1]. Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), has been recently shown to enhance motor functional recovery after ischemic stroke [2,3]. Several ongoing clinical trials are looking at the effect of Fluoxetine when given 2-15 days following stroke onset, including the AFFINITY (Assessment oF FluoxetINe In sTroke recoverY) trial in Australia, the FOCUS (Fluoxetine Or Control Under Supervision) trial in the United Kingdom, and the EFFECTS (Efficacy oF Fluoxetine – a randomizEd Controlled Trial in Stroke) trial in Sweden [4]. Since the majority of patients coming into the hospital are already on statins due to dyslipidemia, the purpose of this study was to examine whether administering fluoxetine at an earlier time-point after stroke onset in combination with a statin could improve outcomes. In this study, we measured infarct volume differences when fluoxetine was administered either 6-12 hours or 20-26 hours after stroke induction in 10-12 month old rats.
Materials and Methods
Subjects
All animal work was approved by the Institutional Animal Care and Use Committee at Wright State University, and all procedures followed were in accordance with institutional guidelines. Animals used were 10-12 month old female Sprague-Dawley rats (generally 300-400 grams), obtained from Harlan Laboratories (Indianapolis, IN) and fed Harlan rat chow. The specific groups are indicated in the Table 1.
| Group |
Gender/ Strain |
Weight at Stroke (mean±SD) |
Drugs (oral) |
Age at Stroke |
| 6-12 hrs. Control |
Female / Sprague Dawley |
329 ± 21.4 gm |
None |
10.5 months |
| 6-12 hrs. FS |
Female/ Sprague Dawley |
332 ± 30.9 gm |
1 mg/kg statin 5 mg/kg fluoxetine |
10.5 months |
| 20-26 hrs. Control |
Female/ Sprague Dawley |
316 ± 19.5 gm |
None |
10.5 months |
| 20-26 hrs. FS |
Female/ Sprague Dawley |
322 ± 27.6 gm |
1 mg/kg statin 5 mg/kg fluoxetine |
10.5 months |
Table 1: Characteristics of treatment groups. FS refers to fluoxetine and statin groups.
Functional tests
Montoya staircase: Rats were fasted overnight and then placed on a restricted diet consisting of 85% of their ad lib rat chow while they were trained to pick up sucrose pellets on the Montoya Staircase [5]. The Montoya Staircase consists of a raised platform with seven stairwells on each side containing sucrose pellets (three per stairwell) on each side of the platform, allowing left and right forelimb function to be assessed. Training occurred during the dark-phase and consisted of 15 minute trials inside the Montoya Staircase with sucrose pellets that have been painted with maple extract; the maple odor helps the rats locate the sucrose pellets on the stair wells. Training generally lasts anywhere from 10-14 days. At the end of the training period, the rat’s pre-stroke performance was determined by the best overall performance (total pellets taken) during the last three days of training. Animals must have learned to pick up at least 9 pellets with both their left and right forepaws or they were excluded from the Montoya Staircase analysis. The majority of animals learned to retrieve between 15-18 pellets with both paws. At 3-5 days post-stroke the rats were tested on the Montoya Staircase again, and their best performance (total pellets taken) was used as a measure of their post-stroke baseline performance. To normalize their performance, we divided the number of pellets taken by each paw post-stroke by the number of pellets taken by each paw pre-stroke. Thus, when the quotient was 1, the animal had fully regained pre-stroke function in a particular limb and when the quotient was less than 1, the animal had a functional deficit in that limb. Animals that failed to reach training criteria on Montoya Staircases were tested on Forelimb Asymmetry.
Forelimb asymmetry: Animals were placed in a clear vertical cylinder and videotaped for 5 minutes. Rats would rear up and touch the walls of the cylinder with their right and left forepaws to explore their environment. Normally, pre-stroke, the majority of rats showed little preference for the right or left paw during this exploration behavior. However, when rats were again tested on post-stroke day 4, they showed a preference for touching the wall with their ipsilateral (right) paw, while they held their left paw back toward their body. In this analysis we looked at the percentage of touches to the wall by right or left paw post-stroke and divided that by their pre-stroke performance percentages. Again, when the quotient was 1, the animal had fully regained pre-stroke function and when the quotient was less than 1, the animal had a functional deficit in that limb. In this test in particular, the increased percentage of right paw use gave us values greater than 1.
Surgery for stroke induction
Anesthesia was induced with 5% isoflurane, and then the animals were maintained on 2-2.5% isoflurane using an anesthesia mask on the stereotactic apparatus (Stoelting Co., USA). The basic surgical procedures are as described in a previous study [6], with the following modifications: two holes were drilled in the skull, with positions relative to Bregma of (0.0 mm A.P., 2.5 mm M.L.) and (1.5 mm A.P., and 2.5 mm M.L.). The needle containing endothelin-1 was inserted into both holes in the skull, then lowered to a depth of 2 mm. One and one-half microliters of endothelin-1 (600 pmol) was injected into each hole over a period of about 5 minutes. These positions were chosen to target the forelimb motor cortex in rats [7].
Treatment conditions
Two different time-points were chosen for initiating 5 mg/ kg fluoxetine and 1 mg/kg simvastatin after stroke induction: 6-12 hours (12 animals per group) and 20-26 hours (8 animals per group). Animals were randomly assigned to either drug or control group after the surgery, but before the baseline stroke deficit was determined using either Montoya Staircase or Forelimb Asymmetry tests. After drug treatment was begun, it was continued daily for a period of 90 days, using voluntary oral administration of the drugs [8]. Briefly, this involves putting the drugs in a 4 gram ball of sugar cookie dough and allowing the animals to eat it. Control animals receive the 4 gram ball of sugar cookie dough with no drugs added.
Histology
The animal was anesthetized with 100 mg/kg, i.p. pentobarbital (Euthasol®), then euthanized by cardiac perfusion with phosphatebuffered saline followed by 4% paraformaldehyde in phosphate buffered saline. The brain was dissected out, the left hemisphere marked, and the brain post-fixed 24 hours in 4% paraformaldehyde in phosphate buffered saline. The brain was then put into a 30% sucrose solution for three days, in preparation for cryosectioning.
Fifty micron coronal slices were cut on a cryostat and collected into phosphate buffered saline. For infarct measurement, these sections were mounted onto gel-subbed slides, then stained with Nissl stain [6], dehydrated and coverslipped with DPX mountant (Sigma-Aldrich).
Infarct measurement
Brightfield images were taken of the sections using a 4X objective, then montaged using Adobe Photoshop 7.0 (Adobe Systems Inc, San Jose CA, USA) into a complete picture of any damaged regions of the brain. Area of the infarct was determined with ImageJ software from NIH. We determined infarct area (mm2) for every third or fourth slice, then multiplied by the slice thickness (mm) and either 3 or 4 respectively, to determine the complete infarct volume in mm3. Individuals determining the infarct volume were blinded to the animal group (control group or fluoxetine-simvastatin group). We lost four brains in the fluoxetine-simvastatin treated group for 6-12 hour administration and two of the fluoxetine-simvastatin treated group (20-26 hour administration) due to damage while cutting the brains on the microtome (shattering), which made the brain slices impossible to mount on slides or damage to brain while dissecting that made determination of complete infarct volume impossible.
Statistics
An unpaired t-test with Welch’s correction for unequal variances was used for statistical comparison of the infarct volumes (Prism 6.0, GraphPad Software Inc. San Diego, USA). Figure 1 shows means and standard error of the means, with individual infarct volumes given by closed symbols clustered around means. Sample sizes are given in the figure legends.
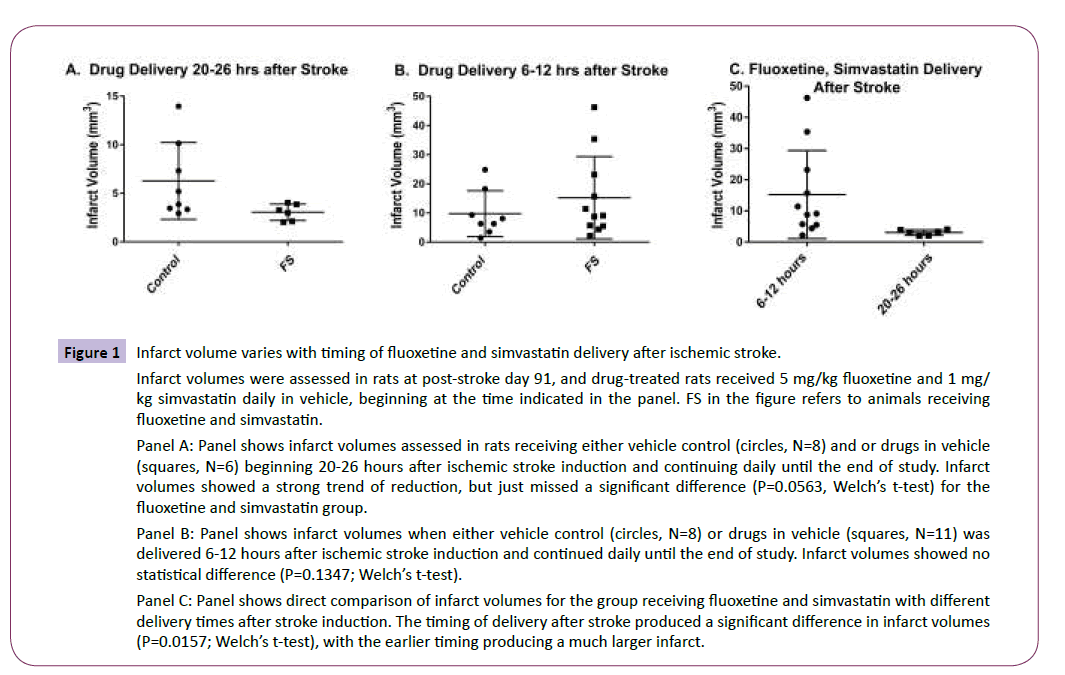
Figure 1: Infarct volume varies with timing of fluoxetine and simvastatin delivery after ischemic stroke.
Infarct volumes were assessed in rats at post-stroke day 91, and drug-treated rats received 5 mg/kg fluoxetine and 1 mg/ kg simvastatin daily in vehicle, beginning at the time indicated in the panel. FS in the figure refers to animals receiving fluoxetine and simvastatin.
Panel A: Panel shows infarct volumes assessed in rats receiving either vehicle control (circles, N=8) and or drugs in vehicle (squares, N=6) beginning 20-26 hours after ischemic stroke induction and continuing daily until the end of study. Infarct volumes showed a strong trend of reduction, but just missed a significant difference (P=0.0563, Welch’s t-test) for the fluoxetine and simvastatin group.
Panel B: Panel shows infarct volumes when either vehicle control (circles, N=8) or drugs in vehicle (squares, N=11) was delivered 6-12 hours after ischemic stroke induction and continued daily until the end of study. Infarct volumes showed no statistical difference (P=0.1347; Welch’s t-test).
Panel C: Panel shows direct comparison of infarct volumes for the group receiving fluoxetine and simvastatin with different delivery times after stroke induction. The timing of delivery after stroke produced a significant difference in infarct volumes (P=0.0157; Welch’s t-test), with the earlier timing producing a much larger infarct.
The online relative risk calculator from the MedCalc site (www. medcalc.org/calc/relative_risk.php) was used to determine the relative risk of the stroke rats developing secondary hemorrhagic stroke due to drug combination administration time and Fisher’s exact test (two-tailed) was used to determine if there was a statistical difference in the occurrence of secondary hemorrhagic infarcts between the two delivery times.
Results
Functional assessment
Female Sprague-Dawley rats (10-12 months old, 300-400 grams) were initially trained to pick up sucrose pellets in the Montoya Staircase [5] and also tested for forelimb bias using the Forelimb Asymmetry Test [6]. We chose old rats as an animal model to better model the human stroke population, as neurogenesis is decreased in both humans and animals with age [9,10], and the drugs chosen would work to increase neurogenesis [11-14]. Three days prior to the stroke, the rats were introduced to the vehicle that would contain their drugs or serve as a vehicle control (4 grams of sugar cookie dough, Pillsbury®) to allow them to get over their avoidance of novel foods; their pre-stroke baseline performance in Montoya Staircase and Forelimb Asymmetry was assessed. To be included in the functional analysis, rats had to pick up at least 9 sucrose pellets with each forepaw in the Montoya Staircase test and show at least a 20% functional deficit. The rats displayed similar post-stroke function in the contralateral paw in each of the time groups: in the 6-12 hours treatment groups, the mean contralateral function was 0.30 ± 0.32 (mean ± SD) in the FS group and 0.33 ± 0.28 in the control group. In the 20- 26 hours treatment groups, the post-stroke mean contralateral function was 0.525 ± 0.31 for the FS group and 0.37 ± 0.27 for the control group. There was no statistical difference in the functional deficits on the contralateral forepaw between the drug and control groups. In some cases, we would see a bilateral deficit, and when those infarcts were examined, the infarcts generally involved a portion of the corpus callosum. Infarcts were induced with endothelin-1 injections at the forelimb motor cortex, and drug treatment with 5 mg/kg fluoxetine and 1 mg/kg simvastatin was begun either 6-12 hours OR 20-26 hours after stroke induction. These drug dosages correlate to approximately a 40 mg fluoxetine dose and a 10 mg dose of simvastatin in humans [15]. This amount of fluoxetine is about twice what is currently being used in on-going clinical trials [3,4].
Exclusions
In this study, we examined infarct sizes in all animals that underwent stroke and also displayed at least a 20% contralateral deficit in the Montoya Staircase or Forelimb Asymmetry. In our endothelin-1 induced stroke method, we positioned a needle with endothelin-1 at a depth of 2 mm from the cortical surface before injecting 600 pmoles of endothelin-1 into the site. This movement of the needle through the cortex sometimes nicks a blood vessel and causes some bleeding, which the endothelin-1 will generally stop for an interval. One of the animals in the control group had substantial bleeding due to this needle damage of blood vessels (Figure 2; Panel A: positive control for hemorrhagic infarcts), which produced secondary hemorrhagic damage in this animal and a huge infarct that spread far anterior and posterior compared to our normal infarcts (total infarct volume of 53.20 mm3). Because this damage was due to a surgical artifact, and not due to the endothelin-1 induced ischemic damage, it was not included in this study. The only other reason that a rat brain was excluded from this analysis was if the brain was damaged either during dissection or during the cryostat sectioning, such that we could not mount the individual slices or could not accurately determine the entire infarct volume.
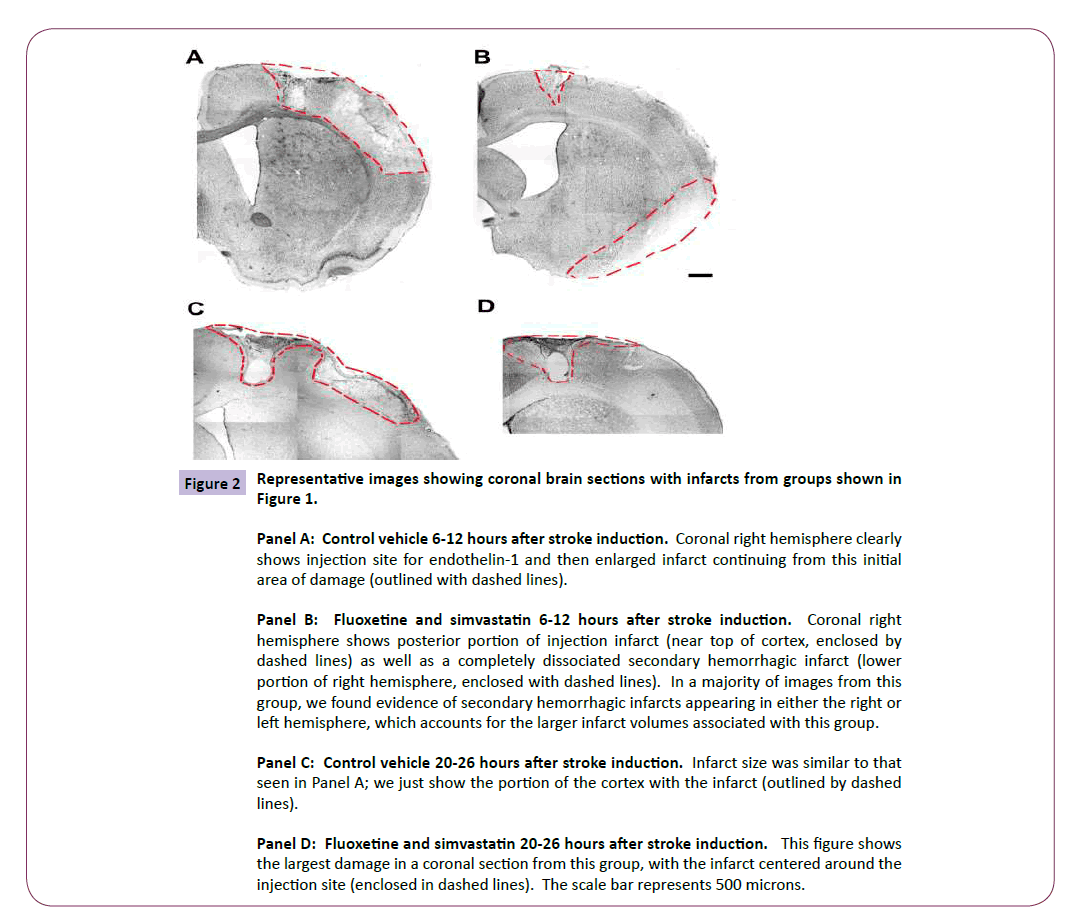
Figure 2: Representative images showing coronal brain sections with infarcts from groups shown in Figure 1.
Panel A: Control vehicle 6-12 hours after stroke induction. Coronal right hemisphere clearly shows injection site for endothelin-1 and then enlarged infarct continuing from this initial area of damage (outlined with dashed lines).
Panel B: Fluoxetine and simvastatin 6-12 hours after stroke induction. Coronal right hemisphere shows posterior portion of injection infarct (near top of cortex, enclosed by dashed lines) as well as a completely dissociated secondary hemorrhagic infarct (lower portion of right hemisphere, enclosed with dashed lines). In a majority of images from this group, we found evidence of secondary hemorrhagic infarcts appearing in either the right or left hemisphere, which accounts for the larger infarct volumes associated with this group.
Panel C: Control vehicle 20-26 hours after stroke induction. Infarct size was similar to that seen in Panel A; we just show the portion of the cortex with the infarct (outlined by dashed lines).
Panel D: Fluoxetine and simvastatin 20-26 hours after stroke induction. This figure shows the largest damage in a coronal section from this group, with the infarct centered around the injection site (enclosed in dashed lines). The scale bar represents 500 microns.
Infarct Volumes vary with timing of Fluoxetine, Statin administration
In Figure 1, infarct volumes were calculated on post-stroke day 91, with animals either receiving fluoxetine and simvastatin (FS in figure, 5 mg/kg, and 1 mg/kg, respectively) or vehicle control, beginning either 6-12 hours or 20-26 hours after stroke induction and continuing daily through post-stroke day 90. In Panel A, we see a strong trend toward a reduction in infarct size in the FS group (3.051 ± 0.3447 mm3, mean ± SEM; squares) compared to control (6.277 ± 0.14 mm3, mean ± SEM; circles) when the fluoxetine and simvastatin drug combination is administered beginning 20-26 hours after the stroke (P=0.0569; Welch’s correction on unpaired t-test with unequal variance), which was an unexpected result. We did not think drug administration this late after stroke induction would have any effect on the infarct volume. The reduction in infarct volume has also correlated with an increase in motor functional recovery, referenced in some of our previous work [6]. We next tested whether an earlier time point for drug delivery would result in further reduction of infarct volume and greater functional recovery, administering the drugs 6-12 hours after stroke induction, a time period over which the majority of stroke patients have been shown to arrive at the hospital [1]. In Panel B, we see that drug delivery at an earlier time point actually resulted in larger infarct volumes (15.24 ± 4.26 mm3; mean ± SEM; squares) which were not statistically different from control infarct volumes (9.775 ± 2.786 mm3; mean ± SEM; circles) (P=0.1347; Welch’s correction for unpaired t-test with unequal variance). In panel C, we directly compared the infarct volumes in the two groups that received fluoxetine and simvastatin, just looking at the effect the difference in delivery time (noted on the X axis) had on the infarct volume. This data was simply taken from panels A and B, re-plotted on the same scale and then compared for statistical difference. Earlier delivery (6-12 hours after stroke) resulted in statistically larger infarcts (circles; mean of 15.24 mm3) compared to later delivery times (20-26 hours after stroke; squares; 3.051 mm3 mean) (P=0.017; Welch’s correction of unpaired t-test with unequal variance). Since this was a surprising result, we examined the infarcts closely to determine why the infarct size was enlarged.
Histological Evidence of Secondary Hemorrhagic Transformation in Rats Receiving Fluoxetine and Statin at an Early Time Point.
In Figure 2, infarcts are shown (outlined with dashed lines) in representative coronal brain sections for each of the relevant groups.
Endothelin-1 injection site was usually clearly seen at the dorsal portion of the cortex as it is generally a hole in the tissue at 91 days post-stroke, since it was the origin of the infarct. In control animals (Panels A and C), we have generally seen additional damage to the right side of this injection point, representing perhaps reperfusion damage when the endothelin-1 was metabolized or eliminated, allowing blood flow to return after approximately 24 hours [16]. Areas with very small surface damage were not included in outlined infarcts just for clarity in this figure, but they were included in the infarct analysis above. In general, we did not see evidence of any secondary hemorrhagic stroke in control animals, whether or not the control vehicle was delivered at 6-12 hours after stroke (Panel A) or 20-26 hours after stroke (Panel C). Panel D shows the infarct size (largest in a series of brain sections) from an animal given fluoxetine and simvastatin 20-26 hours after stroke induction. You only see the original damage associated with the endothelin-1 injection at the core of the infarct, but the rest of the cortex appears to have normal Nissl staining. In contrast to control animals, there does not appear to be damage to the right of the original site, perhaps indicating that reperfusion damage was minimized. Alternatively, the fluoxetine and simvastatin are both known to increase growth factors in the brain, and we have shown in a previous study [6] that one of these growth factors, brain derived neurotrophic factor (BDNF), is elevated around the site of the original infarct; this may allow tissue around the original infarct to survive.
Out of 12 control animals with vehicle delivered at 6-12 hours, we saw two that had possible signs of secondary hemorrhagic infarcts (infarcts were located either far anterior or far posterior and dissociated away from the original infarct). One of these substantial bleeding occurred when lowering the needle into the cortex, so was an artifact of the surgical procedure.
An example of this is shown in Figure 3, panel A, illustrating the extensive bleed from surgical artifact compared to a possible secondary hemorrhagic transformation. One control animal did show some posterior lateral damage, which may have been caused by a secondary hemorrhagic infarct, so we did include this animal when we compared relative risk of secondary hemorrhagic infarcts. In contrast, when we examined fluoxetine and simvastatin delivery 6-12 hours after stroke (Panel B), we found more evidence of secondary hemorrhagic stroke, clearly dissociated from the original endothelin-1 injection site (e.g. ventral location, location in other hemisphere, far anterior or posterior to original injection site): Six of the original 11 animals in the early delivery FS group showed signs of secondary infarcts. The relative risk was calculated to be 4.36 (95% CI = 0.64-29.5) for these small groups (6 positive outcomes and 5 negative outcomes) for the treated group to have a bad outcome compared to control (1 positive and 7 negative).
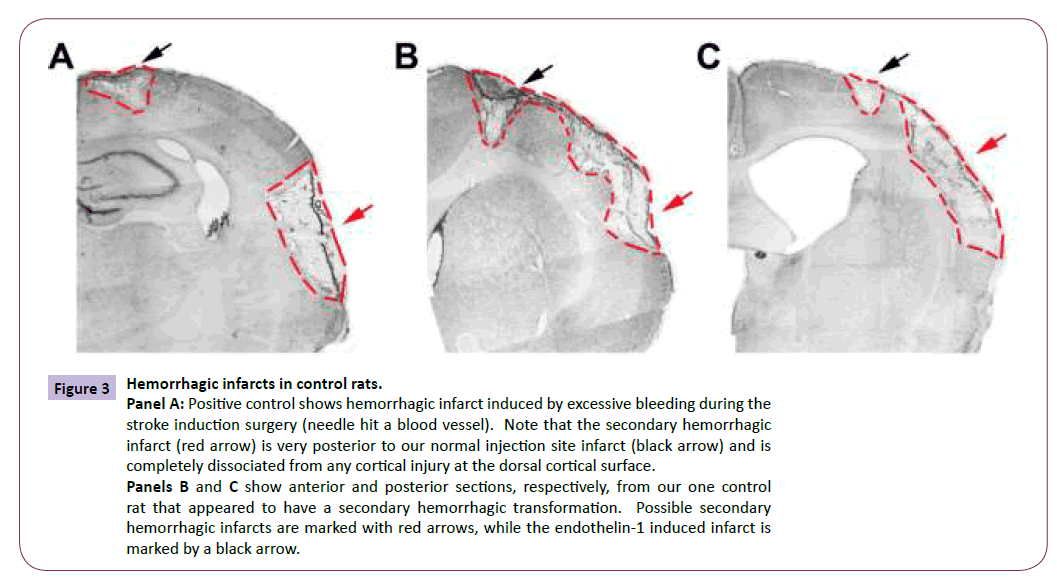
Figure 3: Hemorrhagic infarcts in control rats.
Panel A: Positive control shows hemorrhagic infarct induced by excessive bleeding during the stroke induction surgery (needle hit a blood vessel). Note that the secondary hemorrhagic infarct (red arrow) is very posterior to our normal injection site infarct (black arrow) and is completely dissociated from any cortical injury at the dorsal cortical surface.
Panels B and C show anterior and posterior sections, respectively, from our one control rat that appeared to have a secondary hemorrhagic transformation. Possible secondary hemorrhagic infarcts are marked with red arrows, while the endothelin-1 induced infarct is marked by a black arrow.
Discussion
A number of animal studies have looked at fluoxetine administration Figure 3 in stroked animals or traumatic brain injury animals and many have failed to see any positive effect of the fluoxetine [17-19]. In several of these studies, neurogenesis is examined in the dentate gyrus, with only cursory examination of neurogenesis in lateral ventricles that are very posterior in the brain (to the side of the hippocampus). Also, the delivery method for fluoxetine is generally one which would induce stress in the animal (surgical implantation of an osmotic pump delivering fluoxetine, daily intraperitoneal injections of fluoxetine, oral gavage of fluoxetine, etc.). We have seen increases in neurogenesis in older (10-12 month) rats in the subventricular zone (SVZ) of the anterior lateral ventricles in the presence of fluoxetine when it is given with voluntary oral administration, thus eliminating any stress with administration of the fluoxetine. We also see functional recovery, which correlates well with what is being seen in clinical trials of the drug in stroke patients [2,20] or traumatic brain injury patients [21]. Since stress has an inhibitory effect on neurogenesis and fluoxetine generally has a positive effect [22,23], we believe that care must be taken to eliminate stress when administering the fluoxetine to animal models of disease, which our drug delivery system accomplishes [8].
Both fluoxetine and simvastatin have been shown to increase vascular endothelial growth factor (VEGF) [14,24]. In the early delivery time, the formation of VEGF may cause bleeding because the normal proteins involved in structural support of angiogenesis are not yet available. In a study from Michael Chopp’s lab, early delivery (within 1 hour of induction) of rhVEGF to middle cerebral artery-occluded male Wistar rats showed signs of blood brain barrier leakage and hemorrhagic transformation, while later delivery (48 hours after stroke induction) did not [25]. This study may be the basis for beginning the fluoxetine post-stroke treatment no earlier than the second day post-stroke in ongoing Clinical Trials testing fluoxetine for motor recovery after stroke. We do not have direct evidence for increased VEGF immediately after drug administration yet, but from previous work on these drugs, the preceding was a potential explanation for why we saw hemorrhagic transformation at an earlier time point, but that hemorrhagic transformation disappeared at a later time point, although the drugs were exactly the same at both time points. However, given the results of this study and another study from this lab [6], it appears that giving fluoxetine 20-26 hours after stroke induction not only promotes motor functional recovery, but also reduces the infarct volume significantly compared to giving the fluoxetine and simvastatin within 6-12 hours of stroke induction.
We are currently performing studies where the rats are on the statins prior to stroke induction, and fluoxetine is given at various times after stroke, so that the drug delivery will correlate better with what is seen during clinical trials with fluoxetine. We will directly compare fluoxetine delivery at 6-12 hours, 20-26 hours and 48-54 hours after stroke induction, and note impact on infarct volume at 7 days post-stroke and any trend towards hemorrhagic transformation.
Conclusion
The combination of simvastatin and fluoxetine increased the relative risk (RR= 4.36, 95% CI = 0.64-29.5) of bleeding after ischemic stroke if it is given 6-12 hours after stroke induction in rats. However, delivery of simvastatin and fluoxetine 20-26 hours after ischemic stroke in rats significantly reduces infarct size compared to earlier delivery and shows a strong trend towards infarct reduction compared to controls.
Author Contribution
Maria HH Balch and Moner A Ragas contributed equally to this paper and should both be considered first authors.
Source of Funding
This work was supported by a Women in Science Giving Circle Grant to Adrian M. Corbett; an Original Work Grant to Moner A. Ragas from the Graduate Student Assembly at Wright State University, and internal support from the Vice President for Research and Graduate Studies at Wright State University, Dr. Jack Bantle, given to Adrian M. Corbett.
Disclosures:
None
7363
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, et al. (2012) Heart Disease and Stroke Statistics--2013 Update A Report From the American Heart Association. Circulation.
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, et al. (2011) Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol.
- Mead GE, Hsieh CF, Lee R, Kutlubaev M, Claxton A, et al. (2013) Selective serotonin reuptake inhibitors for stroke recovery: a systematic review and meta-analysis. Stroke44:844-550.
- Mead GE, Hsieh CF, Hackett M (2013) Selective serotonin reuptake inhibitors for stroke recovery. Jama.310:1066-7.
- Montoya CP, CampbellHLJ, Pemberton KD, Dunnett SB (1991) The "staircase test": a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods36:219-228.
- Corbett AM, Sieber S, Wyatt N, Lizzi J, Flannery T, et al. (2015) Increasing neurogenesis with fluoxetine, simvastatin and ascorbic Acid leads to functional recovery in ischemic stroke. Recent patents on drug delivery & formulation9:158-166.
- Fonoff ET, Pereira JF, Camargo LV, Dale CS, Pagano RL, et al. (2009) Functional mapping of the motor cortex of the rat using transdural electrical stimulation. Behav Brain Res.202:138-141.
- Corbett A, McGowin A, Sieber S, Flannery T, Sibbitt B (2012) A method for reliable voluntary oral administration of a fixed dosage (mg/kg) of chronic daily medication to rats. Lab Anim46:318-324.
- Kuhn HG, Dickinson AH, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci16:2027-2033.
- Navailles S, Hof PR, Schmauss C (2008) Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol509:372-381.
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci20:9104-9110.
- Marcussen AB, Flagstad P, Kristjansen PE, Johansen FF, Englund U (2008) Increase in neurogenesis and behavioural benefit after chronic fluoxetine treatment in Wistar rats. ActaNeurolScand117:94-100.
- Lu D, Qu C, Goussev A, Jiang H, Lu C, et al. (2007) Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma24:1132-1146.
- Wu H, Lu D, Jiang H, Xiong Y, Qu C, et al. (2008) Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma25:130-139.
- ReaganSS, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology22:659-661.
- Windle V, Szymanska A, GranterBS, White C, Buist R, et al. (2006) An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. ExpNeurol201:324-334.
- Wang Y, Neumann M, Hansen K, Hong SM, Kim S, et al. (2011) Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma28:259-268.
- Windle V, Corbett D (2005) Fluoxetine and recovery of motor function after focal ischemia in rats. Brain Res1044:25-32.
- CouillardDS, Wuertinger C, Kandasamy M, Caioni M, Stadler K, et al. (2009) Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry14:856-864.
- Gaillard R, Mir O (2011) Fluoxetine and motor recovery after ischaemic stroke. Lancet Neurol.10:499; author reply 500-1.
- Horsfield SA, Rosse RB, Tomasino V, Schwartz BL, Mastropaolo J, et al. (2002) Fluoxetine's effects on cognitive performance in patients with traumatic brain injury. Int J Psychiatry Med32:337-344.
- Dwivedi Y, Rizavi HS, Pandey GN (2006) Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience139:1017-1029.
- Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, et al. (2007) Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res85:3574-3585.
- Lee JS, Jang DJ, Lee N, Ko HG, Kim H, et al. (2009) Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J Neurosci29:8493-8505.
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, et al. (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest106:829-838.








