Ahmed Kamal1*
Tennessee Technological University, College of Engineering, MIT Department, PO Box 5003, Cookeville, TN 38505, TN 38505, USA
*Corresponding Author:
Dr. Ahmed Kamal
MIT Department, Tennessee Tech University
Box 5003, Lewis Hall, Cookeville
TN 38505, USA
Tel: 01-931-239-7570
E-mail: AKamal@tntech.edu
Citation: Kamal A. Assessment of Autonomic Function in Children with Autism and Normal Children Using Spectral Analysis and Posture Entrainment: A Pilot Study. J Neurol Neurosci. 2016, 6:3. doi: 10.21767/2171-6625.100037
Received Date: November 10, 2015; Accepted Date: September 28, 2015; Published Date: September 30, 2015
Objective: The dysfunction of the autonomic nervous system investigated in Children with Autism compared with healthy one. Cardiovascular autonomic disturbances are possibly associated with the pathogenesis of Autism in children. The purpose of this study is to use the spectral and coherence analysis of heart rate variability signal (HRV), respiration signal and peripheral blood flow signal (PBF) to assess the autonomic activity of normal children and children with Autism for the clinical usefulness of the applied methods of signal processing and timefrequency analysis for screening and treatment of Autism in children.
Methods: Twenty four children who had Autism and who were not taking any medications and Twenty three age and sex matched controls (Children) were participated in this study at Department of Neurology at Johns Hopkins Hospital, Baltimore, MD, USA and Regional Medical Center, Cookeville, TN, USA. PBF and respiration signals as well as HRV signal derived from Electrocardiogram (ECG) were measured during supine and standing positions. Autopower, cross power and coherence spectra were produced to investigate the sympathetic and parasympathetic activity in both groups.
Results: The results clearly indicate that in children with Autism, the coherence values are less than in control group in both low frequency (LF) and high frequency (HF) bands at coherence spectra between HRV and PBF as well as HRV and respiration in both supine and standing position. Also, the ratio of amplitude at nearly frequency of 0.1 Hz in auto power spectra and coherence spectra is less in Autism group than in matched control (p<0.001).
Conclusion: Autopower and coherence spectra analysis for Children with Autism compared to normal Children seems useful in the assessment of autonomic function for Autism patients. The decreased of autonomic function specially parasympathetic activity in patients with Autism revealed by auto power and coherence spectra analysis is significant and may be related to the effective of treatment of Autism children in this disorder compared to controls. Further studies is needed using other tests and methods of signal analysis to introduce clinical indices for early detection of autonomic function in patients with Autism.
Keywords
Autonomic system; Heart rate variability analysis; Power spectral analysis; Coherence spectra
Introduction
The short term power spectral analysis of HRV signal (heart rate variability) has been used to assess autonomic control of heart rate [1,2]. Impaired autonomic function has been associated with an increased risk of mortality in autonomic dysfunction in randomly selected general populations. Autonomic dysfunction involving both sympathetic and parasympathetic systems has also been demonstrated in different diseases using cardiovascular reflex tests based on heart rate to various stimuli [1-6]. However, the clinical significance and pathophysiology of these findings in many diseases associated with autonomic malfunction are poorly understood. Conventional time and frequency domain analysis techniques based on the linear fluctuation of heart rate insufficient in outline the changes in heart rate dynamics [7,8], therefore, new methods based on system dynamics have been introduced. The combined application of power spectral analysis of heart rate variability (HRV), Peripheral Blood Flow (PBF) and their coherence to assess the autonomic function to patients using standing specially in short term, is used in this study. HRV and PBF signals in healthy subjects manifested a balance between sympathetic and parasympathetic systems [9-12]. A high variability in heart rate and PBF signal means adaptability implying a healthy individual with well-functioning autonomic control mechanisms [9-12]. Conversely, lower variability is often an indicator of less adaptability of the autonomic nervous system. Reduced heart rate variability has emerged as a strong indicator of risk related to adverse events in patients with range of diseases [5-8,13-17]. In fact, HRV analysis is a practical reproducible and considered noninvasive method to detect early autonomic dysfunction and also permitting better quantitative and qualitative evaluation of sympathovagal modulation of cardiovascular function [18]. Studies of autonomic control of autonomic function in Autism specially in children patients are few and their study population were not homogenous [19].
The aim of this study to assess the autonomic function of a series of randomly selected Autism children and were not taking any medication related to Autism using stimulus standing position by evaluating the power spectra and coherence of several signals including HRV, PBF and respiration signals compared with healthy children.
Methods and Patients
The study was performed at Johns Hopkins university hospital, Baltimore, Maryland, USA, over more than one year period. The study group of patients was composed of 24 children newly diagnosed Autism (10 ± 2.43) years and were not taking medication. For each patient, one healthy age and sex matched control children on no medication was selected. In all cases routine physical examination, a 12 lead surface electrocardiographs, routine biochemistry tests (liver and renal function tests), serum electrolytes, basic hematological parameters were obtained and none of the cases had any evidence of cardiovascular or noncardiovascular disease. None of patients had clinical signs of autonomic dysfunction, history of myocardial infraction, arterial hypertension, diabetes or pulmonary disease. The subjects and the control groups during the study used no drugs that could affect the HRV parameters. Therefore, the final group consisted of 24 male patients with Autism (mean 10 ± 2.43 years) and 23 healthy age and sex matched controls (mean 10 ± 1.57 years). All guardians of children agreed to participate in the research prior to their inclusion in the study and the consent of ethical committee was obtained and approved the study protocol. With each subject lying supine on a bed and physiological measuring devices are connected. The breathing signal measured using a thermistor placed on the nose. The ECG is taken from wrists and the ankle (lead II). An infrared plethysmograph placed on finger indicates PBF signal simultaneously for the duration of experiments. All measurements are interfaced to laptop PC and stored in CD. The second phase of experiments entail that all subjects asked to standup and all the signal measured in this position. The duration of measurements in supine and standing position is 10 min.
Generation of HRV signal and estimation of coherence function
Figure 1 illustrates the derivation of HRV signals from ECG. The technique used in this study to produce HRV signals is based on the hardware circuits described by Ahmed et al. [12] to detect R-R interval and produce HRV signals. The HRV signals were then passed through two 12 bit A/D converters at 10 Hz sampling rate and then interfaced to Laptop PC. A/D converters were designed so that the first conversion would occur when the first R wave of ECG was detected. The stored HRV signals of 24 Autism patients and 23 control subjects were then transferred to spectral package prepared by the author at Tennessee Tech University based on Fast Fourier Transform (FFT) or autoregressive method. The package was stored on PC to produce auto-power spectrum, cross power spectrum and coherence spectrum between different signals including HRV, respiration signal and PBF in supine and standing positions. A rank sum test, a nonparametric analog of independent–samples, t-test, was applied to the data of both groups and results indicate patients with Autism are significantly different from control at 0.001 (p>0.001). The estimation of coherence function is explained in Appendix.

Figure 1: Extraction of Heart rate variability signals from ECG.
Results
Table 1 Illustrates the number of subjects (control and Autism patients) exhibiting amplitude entrainment after standing position at Traube–Herring Mayer component (THM) i.e. at frequency at 0.1 Hz expressed in ratio of amplitude at 0.1 Hz at standing to the same frequency (0.1Hz) at Supine position.
Figure 2 represents the typical HRV spectrum for control subject and Autistic patient in standing positions respectively. Similar Figures are obtained for PBF. B Peak represents peak oscillation at 0.1 Hz and R Peak represents Peak oscillation of respiration.
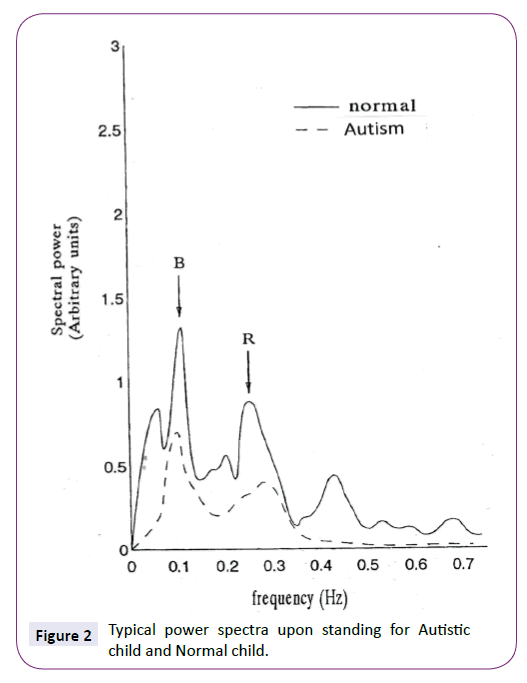
Figure 2: Typical power spectra upon standing for Autistic child and Normal child.
Figure 3 represents the typical coherence spectra between breathing and HRV signals for Autism patient and control subject at standing stimulus.
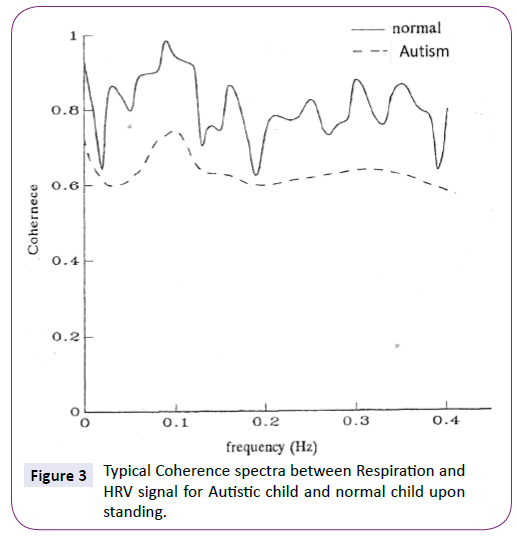
Figure 3: Typical Coherence spectra between Respiration and HRV signal for Autistic child and normal child upon standing.
Discussion
Until now, only some researchers have assessed autonomic function in children with Autism by means of a neurophysiological methods [6,11]. However, this study is directed to use the power spectra and coherence spectra of heart rate variability signals(extracted from Cardiovascular signal ECG) and Peripheral Blood flow signal (PBF) to assess the function of autonomic system in Autism children patients compared with normal subjects. Figure 2 shows the change in blood pressure frequency which oscillates at 0.1 Hz and shown in power spectrum of heart rate variability signals in Figure 2 in the standing position for both Autism patients and normal control subjects. The ratio of amplitude of component of blood pressure at 0.1 Hz between standing and supine positions for control subjects and Autism patients extracted from the autopower spectrum of HRV signal is shown in Figure 2 and demonstrated in Table 1.
Ratio of Amplitudes
B (standing)/B (Supine) |
Number of Healthy Subjects |
Number of Autism Patients |
| <1 |
0 |
0 |
| 1-1.19 |
0 |
14 |
| 1.2-1.39 |
3 |
8 |
| 1.4-1.59 |
15 |
2 |
| 1.6-1.79 |
3 |
0 |
| >1.8 |
2 |
0 |
| Total |
23 |
24 |
Table 1: Number of Subjects (Control and Autism patients) Exhibiting Amplitude Entrainment upon Standing.
Referring to Table 1, it appears for control healthy subjects 15 out of 23 (65%) that have ratios in the range of 1.4-1.59. However, for Autism patients 14 out of 24 (60%) have lower ratios in the range of 1-1.19. This is clear indication of the effect of standing as natural stimulus to autonomic system to mediate the stimulus signal via baroreceptor to the heart [17-19]. Using coherence spectra between HRV and breathing signals are demonstrated in Figure 3. Figure 3 illustrates the low coherence values of HRV for autism patients with respect to control healthy subjects. Once again, this may indicate that autonomic system (both sympathetic and parasympathetic) cannot convey the control oscillations (thermoregulatory oscillation at 0.03 Hz, blood pressure oscillation at 0.1 Hz and respiration oscillation at nearly 0.25Hz) as manifested in coherence spectrum.
In fact, the control group exhibits high values of coherence in both coherence spectrum and Auto power Spectrum as shown in Figures 2 and 3. However, the Autism group shows less coherence values especially around respiration oscillation (nearly around 0.25 Hz) as illustrated in Figure 3. This may be attributed to dysfunction of autonomic nervous system especially parasympathetic part which normally mediated respiration signal to heart and other physiological signal in the body [9-12,15-23].
In Fact, the introduction of coherence values in this study to assess the autonomic function of Autism children patients and control healthy subjects may be helpful in using the coherence value as diagnostic medical index for autonomic function assessment [9,21-23]. Therefore one should distinguish this diagnostic index as coherence to test the autonomic function which could be performed in an ambulatory setting in every patient. The patients with diagnosed as low coherence (dysautonomia) should be carefully observed and specific drugs ordered such as betablockers or angiotensin-converting enzyme inhibitors to prevent morality due to autonomic dysfunction. The novelty of this study is combined different signal including HRV, PBF and respiration signals with coherence and auto power spectra compared with one signal EEG analysis using power spectra and coherence.
In summary, autonomic dysfunction revealed by coherence and posture entrainment in this pilot study is present uncommonly in patients with Autism.
The use of coherence value as medical index in diagnosis of dysfunction autonomic system in Autism need further study. We suggest that all patients with suspicion of Autism specially in children can investigated autonomic dysfunction should be under special cardio logical care.
Conclusion and Future Work
This study pointed significance of using the spectral analysis Method and Coherence index in assessing the function of autonomic nervous system for both groups.
However, further study is required to investigate more patients as well as using other quantitative methods to identify the prognosis of autonomic function with duration of Autism in children.
Acknowledgements
The author would like to appreciate the cooperation and collaboration of Department of neurology at Johns Hopkins Hospital, Baltimore, MA, USA and Massachusetts General Hospital, Boston, MA, USA for facilitating the measurement of physiological signals.
The processing and analysis of the signals as well as developing the algorithms were carried out at Tennessee Tech University, Cookeville, TN 38501, USA.
7297
References
- Ming X, Patel R, Kang V, Chokroverty S, Julu PO (2015)Respiratoryand autonomic dysfunction in children with autism spectrum disorders. Brain Dev.
- Klusek J, Roberts JE, Losh M (2015) Cardiac autonomic regulation in autism and Fragile X syndrome: a review.Psychol Bull141:141-175.
- Casanova MF, Hensley MK, Sokhadze EM, El-Baz AS, Wang Y, et al. (2014) Effects of weekly low-frequency rTMS on autonomic measures in children with autism spectrum disorder.Front Hum Neurosci 8:851.
- McCarthy M (2014)Autism diagnoses in the US rise by 30%, CDC reports. BMJ348:g2520.
- Shahrestani S, Stewart EM, Quintana DS, Hickie IB, Guastella AJ (2014) Heart rate variability during social interactions in children with and without psychopathology: a meta-analysis.J Child Psychol Psychiatry55:981-989.
- EilamST, Xu P, Cao M, Gu X, Van Dam NT (2014) Abnormal autonomic and associated brain activities during rest in autism spectrum disorder. Brain137:153-171.
- McGinnis WR, Audhya T, Edelson SM (2013) Proposed toxic and hypoxic impairment of a brainstem locus in autism. Int J Environ Res Public Health10(12).
- Patriquin MA, Lorenzi J, Scarpa A (2013) Relationship between respiratory sinus arrhythmia, heart period, and caregiver-reported language and cognitive delays in children with autism spectrum disorders. ApplPsychophysiol Biofeedback38:203-207.
- Malliani A, Pagani M, Lombardi F, Cerutti S (1991) Cardiovascular neural regulation explored in the frequency domain.circulation 84: 482-492.
- Baselli G,cerutti S, LiberatiD,LombardiF,Malliani A, et al. (1986) Spectral and cross spectralanalysis of heart rate and arterial blood pressure variability signals. Comp Biomed Res 19:520-534.
- Pagani M,Lombardi F, Guzzetti S, Malliani A (1986) Power spectral analysis of heart rate and arterial pressure variability’s as marker of sympathovagal interaction in man and consciousdog. CircRes 59:178-193.
- Ahmed K, Harness JB ,Mearns AJ (1982) Respiratory control of heart rate.Eur J Applied Physiol 50: 95-104.
- Kushki A, Drumm E, PlaMobarak M, Tanel N, Dupuis A, et al. (2013) Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS One 8(4).
- Cheshire WP (2012) Highlights in clinical autonomic neuroscience: new insights into autonomic dysfunction in autism. AutonNeurosci171:4-7.
- Chang MC, Parham LD, Blanche EI, Schell A, Chou CP, et al. (2012) Autonomic and behavioral responses of children with autism to auditory stimuli Am J OccupTher66:567-576.
- Roberts JE, Tonnsen B, Robinson A, Shinkareva SV (2012) Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. Am J Intellect DevDisabil117:90-102.
- Kostyuk N, Rajnarayanan RV, Isokpehi RD, Cohly HH (2010) Autism from a biometric perspective.Int J Environ Res Public Health7:1984-1995.
- Ming X, Bain JM, Smith D, Brimacombe M, Gold von SG, et al. (2011) Assessing autonomic dysfunction symptoms in children: a pilot study.J Child Neurol 26(4).
- Ramachandran VS, Oberman LM (2006) Broken mirrors: a theory of autism.Sci Am295:62-69.
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML (2012) Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005 Oct;27(7):509-16. Erratum in: Brain Dev34:704.
- Hirstein W, Iversen P, Ramachandran VS (2001) Autonomic responses of autistic children to people and objects. ProcBiolSci 268:1883-1888.
- Palkovitz RJ, Wiesenfeld AR (1980) Differential autonomic responses of autistic and normal children.J Autism DevDisord10:347-360
- Pumprla J, Howarka K, Groves K (2002) Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol84:1-8.








