Keywords
Lichens; Bactericidal; Disc-diffusion; Natural extracts
Introduction
The search for novel bioactive secondary metabolites is of primary concern since infectious diseases are continuously emerging and reemerging [1]. Pharmaceutical industries are forced to develop new pharmacological molecules. Similar to higher plants, lichens are considered as potential source of novel biologically active compounds [2]. Historically, a large portion of the world's medicine has been derived from plants and fungi. Important antibiotics such as penicillin are derived from fungi. Lichens are another type of organism that may hold the potential for medical exploration [3]. Interest in the antibiotic potential of lichen compounds was extremely high during the post- World War II era through the end of the 1950's. Once again there is an interest in the potential uses of antibiotics derived from lichens, as they may be a valuable source of antibiotics for the pharmaceutical industries in the future [3]. Antibiotic properties of the lichens are of special interest to the scientists [4,5]. According to one estimate, 50% of all lichens have antibiotic properties [5,6]. Burkholder was pioneer initiating research on lichens as antibacterial agents. He tested 42 lichens for antibiotic property and 27 were reported to inhibit growth of bacteria [5,7-15]. Based on wide screening of antimicrobial activity of lichen extracts, it seems that bacterial inhibitions can vary within the lichen extract, solvent used for extraction and bacteria tested [15]. The emergence of drug-resistant bacterial strains has currently led to severe clinical complications. Hence, new antimicrobial agents are urgently needed and as the search is being extended to new sources, lichens are clearly an interesting source of compounds in pharmaceutical research [8]. Lichens are known to synthesize variety of secondary metabolites with wide range of biological activities [10]. Yamamoto [9] had reviewed the folklore and pharmacological studies on biological activities of lichen.
Materials and Methods
Lichen materials
Lichen species Heterodermia leucomelos(L.) Poelt. (Voucher No. 34755), Cladonia subradiata (Vainio) Sandst. (Voucher No. 34756), Parmotrema tinctorum (Delise ex Nyl.) Hale (Voucher No. 34757), Leptogium sp. (Ach.) Gray. (Voucher No. 34758), Parmotrema crinitum Choisy (Voucher No. 34759), Herpothallon sp. Tobler (Voucher No. 34760), Parmotrema reticulatum (Taylor) M. Choisy (Voucher No. 34761) and Ramalina celastri (Sprengel) Krog & Swinscow (Voucher No. 34762) were collected from Ooty, India and a part of each identified species are preserved in NBRI- Lucknow, India.
Microorganisms: Bacterial strains Salmonella typhimurium (NCIM 2501), Pseudomonas aeruginosa (NCIM 2200), Bacillus subtilis (NCIM 2063), Escherichia coli (NCIM 2065), Proteus vulgaris (NCIM 2027) and Staphylococcus aureus (NCIM 2654), were used in this study. All the microorganisms had been obtained from National Collection of Industrial Microorganisms (NCIM) at National Chemical Laboratory, Pune, India and were maintained on recommended Muller-Hinton medium.
Extraction
Lichen materials were washed, air-dried and grinded for the extraction process. The powdered lichen thallus were placed in a thimble and extracted by soxhlet apparatus using four organic solvent; hexane, ethyl acetate, methanol and ethanol.
Determination of microbial concentration
In order to determine the suitable concentration of microbial culture for antimicrobial activity of lichen extract, microorganisms were inoculated in Muller-Hinton broth and incubated at 34°C for 24 hrs. After completion of incubation period microorganisms were serially diluted in 0.86% sodium chloride saline in the concentration of 10-2, 10-4, 10-6, 10-8. From each concentration 100 μl and 10 μl of microbial suspension were inoculated in separate petri plates containing Muller Hinton agar media and colony-forming units (CFU/ mL) were calculated.
Bactericidal activity using agar-disc diffusion method
Preliminary antimicrobial activity was done by disc diffusion method [11,12]. In this method two sets of microbial suspension; 6 Log10 CFU/mL (10 μl) and 9 Log10 CFU/mL (100 μl) with 10-2 concentration (as the most suitable one) were used to examine efficacy of lichen extracts on different concentration of bacteria. Muller Hinton agar plates were inoculated by microbial suspension and spread completely by glass spreader, then kept for 30 min for adsorption of microorganism on the surface of the media. Whatman filter paper No. 1 were used to prepare 0.6 cm diameter discs and soaked in different lichen extract (200 μg/ml) and implanted on surface of media [14]. All the petri plates were incubated at 34°C for 24 hrs. After completion of incubation period, zone of inhibition were measured. Tetracycline (30 μg/ml), Streptomycin (10 μg/ml), Ofloxacin (5 μg/ml) and Cefixime (5 μg/ml) were used as positive controls which are most potent standard antibiotics available against tested bacteria to compare lichen extracts with highly effective antibiotics [13].
Results
The suitable concentration of microbial cultures to grow fully on Muller-Hinton agar was found to be 10-2 in 0.86% sodium chloride saline. Therefore, 10 and 100 μl of 10-2 microbial suspension concentration was taken and after 24 hrs of inoculation, colony-forming units (CFU/ mL) were calculated. For all the inoculated organisms, 100 μl had 9 Log10 CFU/mL and 10 μl had 6 Log10 CFU/mL of microbial density. For susceptibility test by agar disc diffusion method, both volumes were used in two different sets to examine the efficacy of lichen extract on higher number of bacteria. But for all further studies, 10 μl of 10-2 concentration (106 CFU/mL) was used as it was found more suitable for antimicrobial experiments (Plates 1-7).
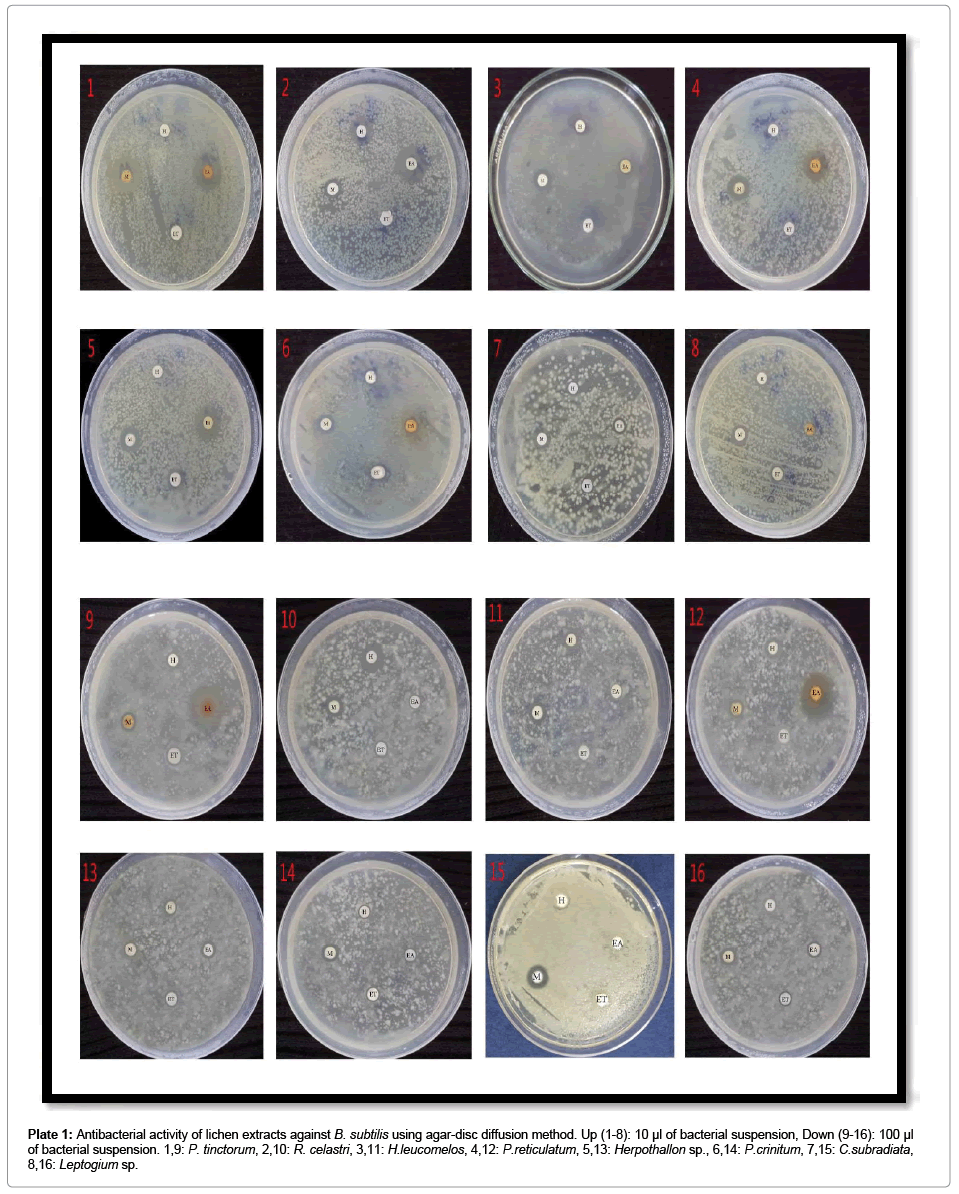
Plate 1: Antibacterial activity of lichen extracts against B. subtilis using agar-disc diffusion method. Up (1-8): 10 μl of bacterial suspension, Down (9-16): 100 μl of bacterial suspension. 1,9: P. tinctorum, 2,10: R. celastri, 3,11: H.leucomelos, 4,12: P.reticulatum, 5,13: Herpothallon sp., 6,14: P.crinitum, 7,15: C.subradiata, 8,16: Leptogium sp.
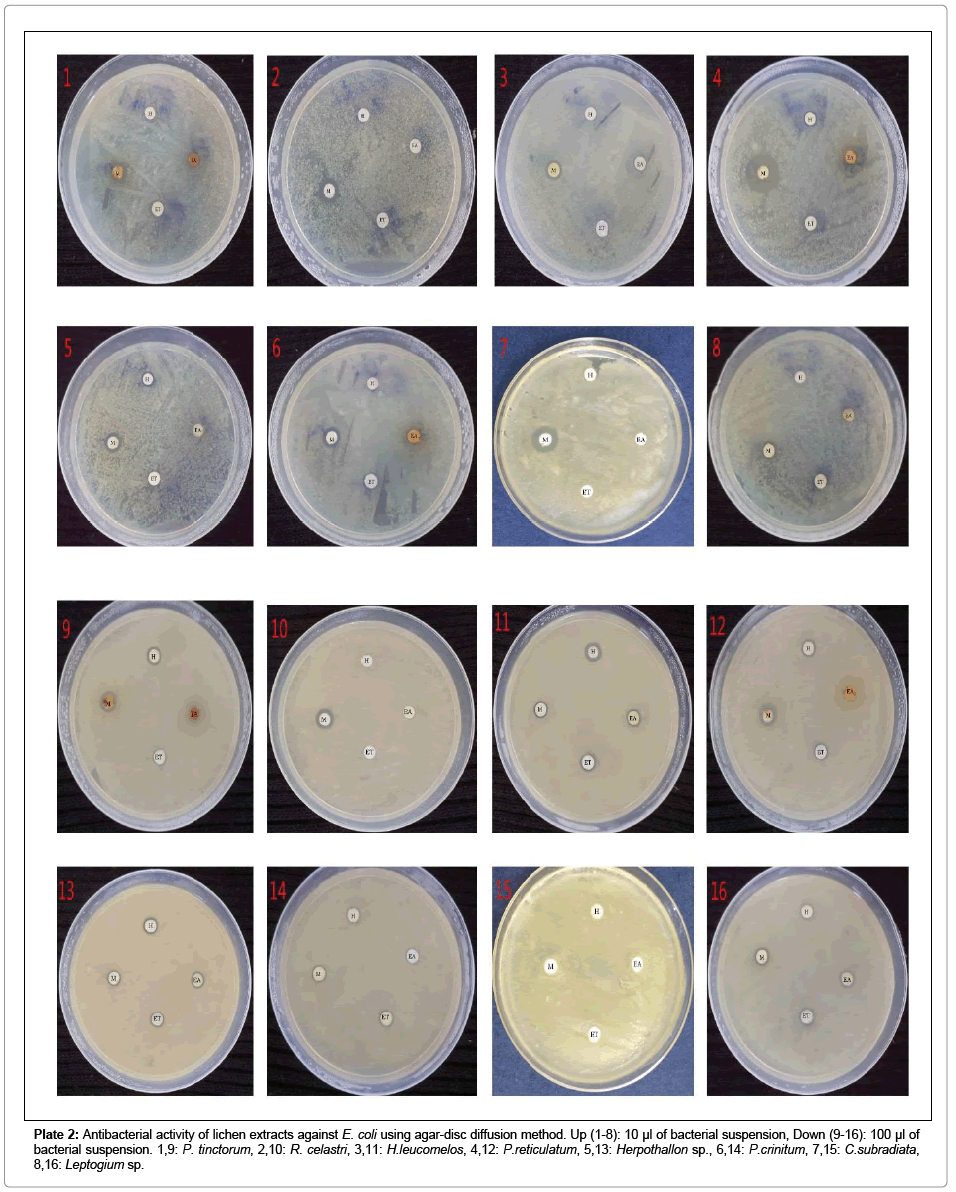
Plate 2: Antibacterial activity of lichen extracts against E. coli using agar-disc diffusion method. Up (1-8): 10 μl of bacterial suspension, Down (9-16): 100 μl of bacterial suspension. 1,9: P. tinctorum, 2,10: R. celastri, 3,11: H.leucomelos, 4,12: P.reticulatum, 5,13: Herpothallon sp., 6,14: P.crinitum, 7,15: C.subradiata, 8,16: Leptogium sp.
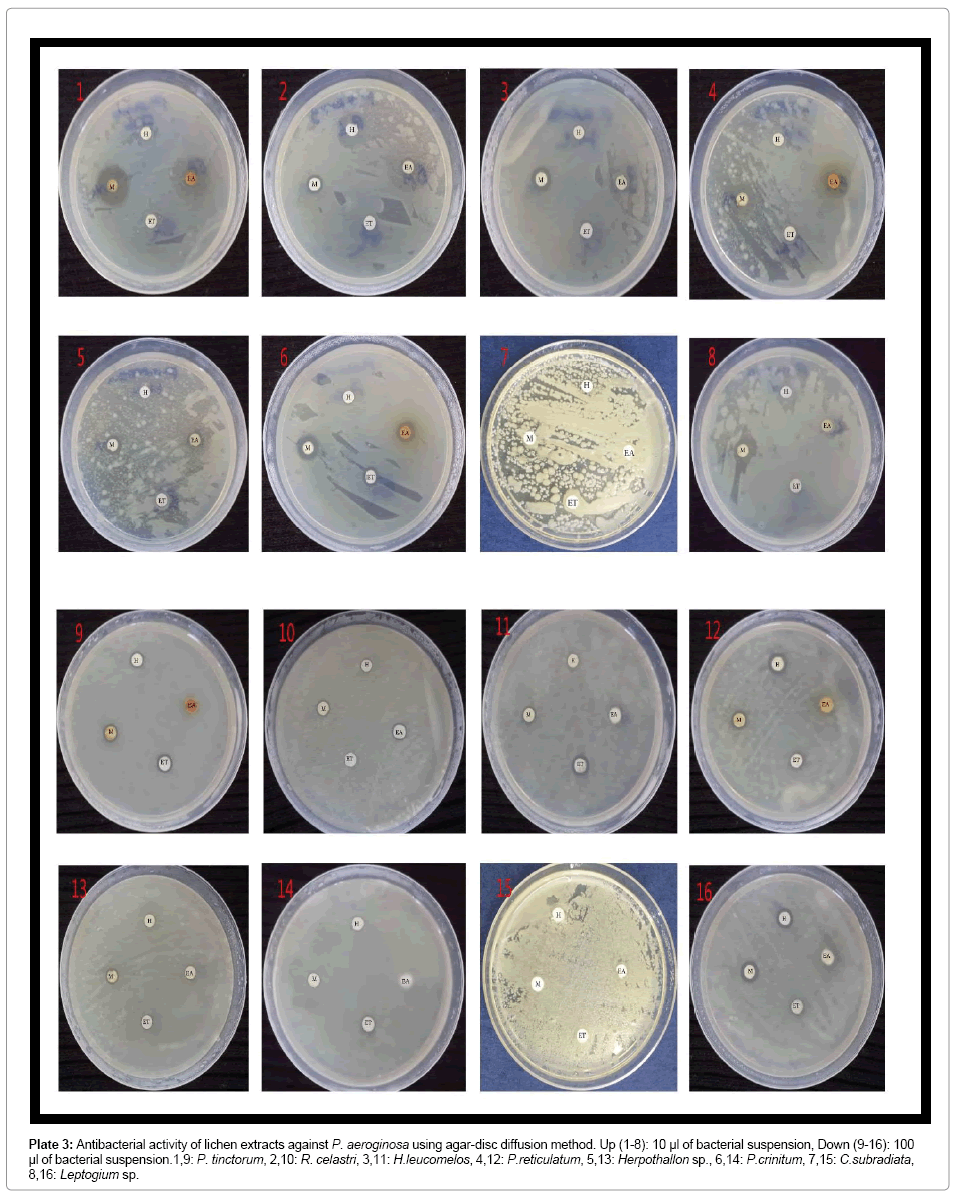
Plate 3: Antibacterial activity of lichen extracts against P. aeroginosa using agar-disc diffusion method. Up (1-8): 10 μl of bacterial suspension, Down (9-16): 100 μl of bacterial suspension.1,9: P. tinctorum, 2,10: R. celastri, 3,11: H.leucomelos, 4,12: P.reticulatum, 5,13: Herpothallon sp., 6,14: P.crinitum, 7,15: C.subradiata, 8,16: Leptogium sp.
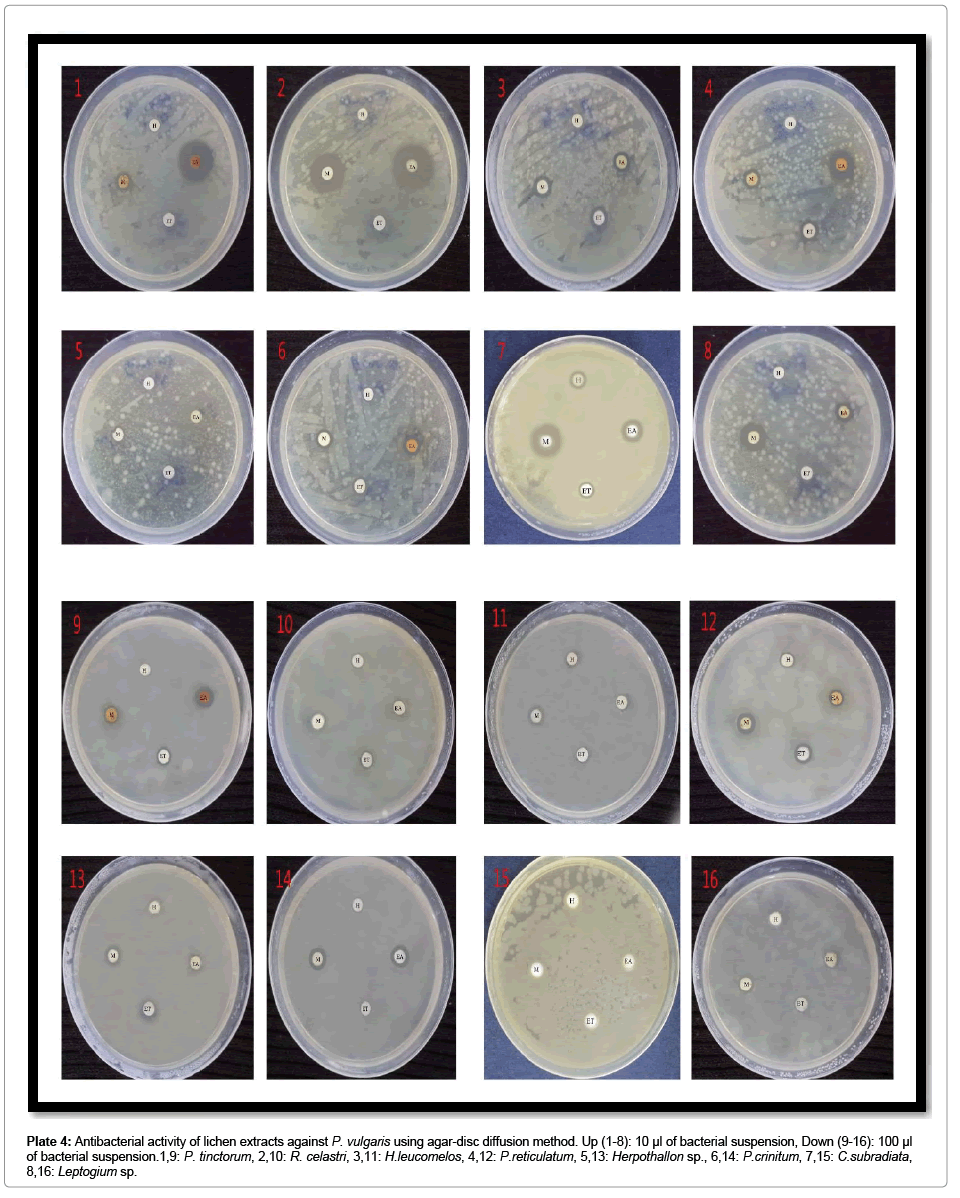
Plate 4: Antibacterial activity of lichen extracts against P. vulgaris using agar-disc diffusion method. Up (1-8): 10 μl of bacterial suspension, Down (9-16): 100 μl of bacterial suspension.1,9: P. tinctorum, 2,10: R. celastri, 3,11: H.leucomelos, 4,12: P.reticulatum, 5,13: Herpothallon sp., 6,14: P.crinitum, 7,15: C.subradiata, 8,16: Leptogium sp.
Plate 5: Antibacterial activity of lichen extracts against S. aureus using agar-disc diffusion method.Up (1-8): 10 μl of bacterial suspension, Down (9-16): 100 μl of bacterial suspension.1,9: P. tinctorum, 2,10: R. celastri, 3,11: H.leucomelos, 4,12: P.reticulatum, 5,13: Herpothallon sp., 6,14: P.crinitum, 7,15: C.subradiata, 8,16: Leptogium sp.
Plate 6: Antibacterial activity of lichen extracts against S. typhimurium using agar-disc diffusion method. Up (1-8): 10 μl of bacterial suspension, Down (9- 16): 100 μl of bacterial suspension. 1,9: P. tinctorum, 2,10: R. celastri, 3,11: H.leucomelos, 4,12: P.reticulatum, 5,13: Herpothallon sp., 6,14: P.crinitum, 7,15: C.subradiata, 8,16: Leptogium sp.
Plate 7: Standard antibiotic activity (1) B. subtilis (2) E. coli (3) P.aeroginosa (4) P. vulgaris (5) S.aureus (6) S. thyphimurium. Left 100 μl microbial suspension, Right: 10 μl microbial suspension. (T: Tetracycline, S: Streptomycin, O: Ofloxacin, C: Cefixime).
Preliminary study on antibiotic activity of lichen extracts showed overall good efficacy of tested extracts on 6 Log10 CFU/mL of bacterial suspensions and moderate activity on 9 Log10 CFU/mL. Methanol followed by ethyl acetate extracts were the most bactericidal in nature. The order of each sample for their antibiotic activity according to average of their zone of inhibition was as follow: P. tinctorum (maximum ZOI: 32 mm) followed by P. reticulatum (maximum ZOI: 28 mm), R. celastri (maximum ZOI: 22 mm), Herpothallon sp. (maximum ZOI: 26 mm), H. leucomelos (maximum ZOI: 21 mm), P. crinitum (maximum ZOI: 18 mm), Leptogium sp. (maximum ZOI: 17 mm) and C. subradiata (maximum ZOI: 16 mm) (Table 1; Figure 1).
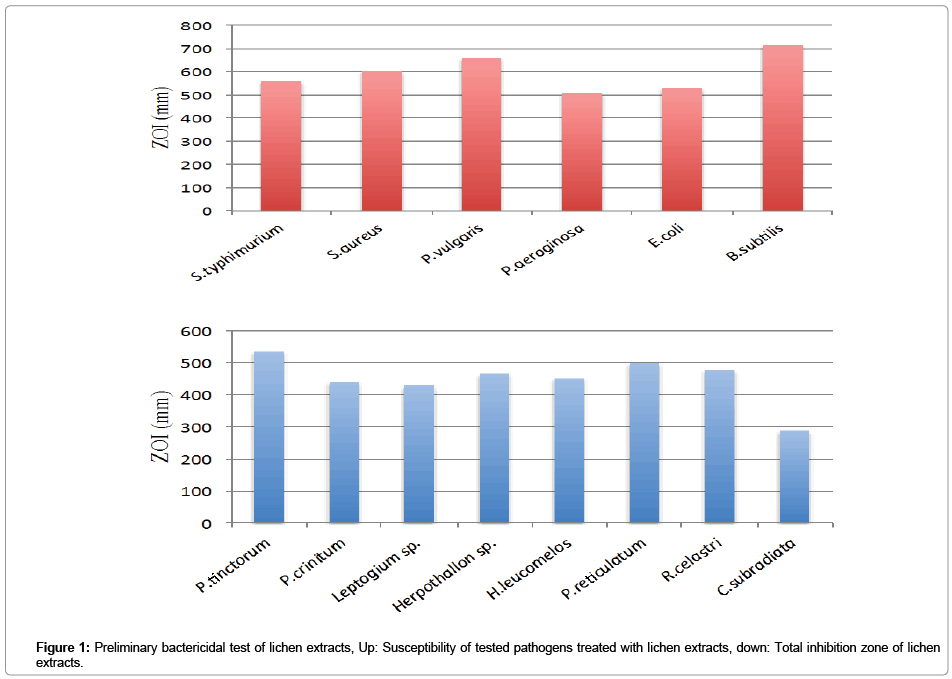
Figure 1: Preliminary bactericidal test of lichen extracts, Up: Susceptibility of tested pathogens treated with lichen extracts, down: Total inhibition zone of lichen extracts.
| Lichen species solvent extract |
B.subtilis |
E.coli |
P.aeroginosa |
P.vulgaris |
S.aureus |
S.typhi |
| Volume (µl) |
100 |
10 |
100 |
10 |
100 |
10 |
100 |
10 |
100 |
10 |
100 |
10 |
| P.tinctorum |
|
| Hexane |
9 |
7 |
10 |
10 |
9 |
8 |
7 |
- |
9 |
8 |
7 |
8 |
| Ethyl acetate |
23 |
28 |
- |
9 |
7 |
10 |
15 |
28 |
7 |
10 |
7 |
15 |
| Methanol |
12 |
32 |
10 |
16 |
9 |
18 |
10 |
12 |
12 |
12 |
7 |
14 |
| Ethanol |
10 |
10 |
- |
12 |
8 |
7 |
8 |
7 |
16 |
16 |
10 |
15 |
| P. crinitum |
|
|
|
|
|
|
| Hexane |
9 |
10 |
7 |
10 |
7 |
7 |
7 |
10 |
- |
- |
8 |
8 |
| Ethyl acetate |
11 |
12 |
7 |
8 |
- |
12 |
11 |
16 |
- |
18 |
7 |
7 |
| Methanol |
12 |
14 |
7 |
16 |
7 |
15 |
12 |
15 |
8 |
9 |
9 |
14 |
| Ethanol |
9 |
11 |
7 |
10 |
8 |
11 |
7 |
14 |
7 |
8 |
7 |
- |
| Leptogium sp. |
|
|
|
|
|
|
| Hexane |
- |
10 |
7 |
8 |
10 |
9 |
7 |
7 |
- |
- |
7 |
7 |
| Ethyl acetate |
10 |
- |
8 |
8 |
7 |
7 |
8 |
13 |
10 |
11 |
8 |
11 |
| Methanol |
12 |
13 |
10 |
15 |
10 |
12 |
10 |
17 |
9 |
14 |
8 |
10 |
| Ethanol |
10 |
10 |
7 |
8 |
7 |
8 |
8 |
16 |
10 |
9 |
8 |
12 |
| Herpothallon sp. |
|
|
|
|
|
|
| Hexane |
7 |
7 |
10 |
10 |
7 |
7 |
7 |
7 |
7 |
7 |
8 |
7 |
| Ethyl acetate |
10 |
26 |
8 |
10 |
7 |
10 |
7 |
7 |
8 |
7 |
7 |
12 |
| Methanol |
9 |
20 |
10 |
16 |
10 |
14 |
12 |
12 |
8 |
14 |
7 |
14 |
| Ethanol |
9 |
11 |
8 |
10 |
7 |
7 |
9 |
7 |
9 |
9 |
8 |
15 |
| H.leucomelos |
|
| Hexane |
10 |
10 |
10 |
7 |
8 |
7 |
7 |
11 |
7 |
13 |
7 |
8 |
| Ethyl acetate |
8 |
8 |
8 |
10 |
7 |
7 |
8 |
14 |
7 |
11 |
8 |
8 |
| Methanol |
15 |
16 |
9 |
12 |
9 |
13 |
12 |
16 |
8 |
7 |
7 |
15 |
| Ethanol |
- |
- |
8 |
10 |
10 |
7 |
8 |
14 |
10 |
21 |
7 |
8 |
| P.reticulatum |
|
|
|
|
|
|
| Hexane |
12 |
7 |
9 |
11 |
9 |
7 |
8 |
8 |
10 |
12 |
11 |
8 |
| Ethyl acetate |
20 |
22 |
8 |
9 |
7 |
7 |
10 |
20 |
8 |
8 |
7 |
12 |
| Methanol |
8 |
20 |
12 |
28 |
10 |
16 |
14 |
15 |
8 |
12 |
9 |
7 |
| Ethanol |
- |
- |
8 |
11 |
7 |
7 |
11 |
12 |
9 |
10 |
7 |
7 |
| R. celastri |
|
|
|
|
|
|
| Hexane |
17 |
20 |
7 |
- |
8 |
18 |
7 |
12 |
9 |
9 |
7 |
7 |
| Ethyl acetate |
9 |
25 |
7 |
7 |
10 |
12 |
7 |
20 |
7 |
20 |
7 |
7 |
| Methanol |
13 |
15 |
12 |
12 |
10 |
13 |
9 |
22 |
7 |
11 |
7 |
7 |
| Ethanol |
8 |
7 |
- |
7 |
7 |
9 |
7 |
7 |
7 |
7 |
7 |
7 |
| Cladonia subradiata |
|
|
|
|
|
|
|
|
|
|
|
|
| Hexane |
7 |
8 |
- |
- |
- |
- |
- |
12 |
8 |
16 |
7 |
10 |
| Ethyl acetate |
- |
8 |
- |
- |
- |
- |
- |
8 |
9 |
16 |
- |
12 |
| Methanol |
14 |
15 |
- |
15 |
- |
- |
- |
17 |
11 |
16 |
12 |
16 |
| Ethanol |
- |
8 |
- |
- |
- |
- |
- |
9 |
- |
16 |
7 |
10 |
| Standard antibiotics |
|
|
|
|
|
|
| Tetracycline |
30 |
32 |
27 |
28 |
19 |
25 |
22 |
23 |
32 |
30 |
- |
42 |
| Streptomycin |
- |
- |
26 |
26 |
29 |
42 |
30 |
30 |
27 |
28 |
- |
- |
| Ofloxacin |
35 |
36 |
38 |
42 |
42 |
50 |
42 |
50 |
42 |
46 |
- |
45 |
| Cefixime |
- |
- |
24 |
25 |
- |
18 |
- |
- |
- |
16 |
- |
- |
Table 1: Antibacterial activity (zone of inhibition, mm) of various solvent fractions of lichen species and standard antibiotics with two different concentrations (10, 100 μl).
As far as susceptibility of bacteria is concerned, B. subtilis was the most sensitive bacteria by tested extracts and P. aeruginosa was the least (Figure 1). Tetracycline was most potent positive standard antibiotic but still in 9 Log10 CFU/mL of S. typhimurium did not have any effect whereas all methanolic tested lichen extracts in same concentration of this bacteria were effective (Table 1).
Conclusion
Extensive literature survey indicates that lichens are good source of antibiotic secondary metabolites and can be utilized to cure various infectious diseases. Hence, here bactericidal activities of thirty-two extracts from eight lichen species were investigated. According to the results, lichen extracts had demonstrated moderate to highly potent antibacterial activity with maximum inhibition in methanol extracts, therefore, it was concluded that these methanolic lichen species might be used further for in vivo studies due to their broad-spectrum bactericidal activity.
18665
References
- Santiago KAA, Borricano JNC, Canal JN, Marcelo DMA, Perez MCP, et al. (2010) Antibacterial activity of fruticose lichens collected from selected sites in Luzon Island, Philippines. Philippine Science Letters3: 18-29.
- MitrovicÃÂ T, Stamenkovic S, CvetkovicÃÂ V, Nikolic M, TošicÃÂ S, et al. (2011) Lichens as source of versatile bioactive compounds. Biologica Nyssana2: 1-6.
- Crockett M, Kageyama S, Homen D, Lewis C, Osborn J, et al. (2003) Antibacterial properties of four pacific Northwest lichens. Lichenology group, Oregon State University.
- Lawrey JD (1986) Biological role of lichen substances. The Byrologist 89: 111-122.
- Malhotra S, Subban R, Singh A (2007)Lichens- Role in Traditional Medicine and Drug Discovery. The Internet Journal of Alternative Medicine5: 2.
- Sharnoff SD (1997) Lichens and people. For a bibliographical database of the human uses of lichens.
- Burkholder PR (1944) Antibiotic activity of lichens. Proceedings of the National Academy of Sciences of theUSA 30: 250-255.
- Boustie J, Grube M (2005) Lichens-a promising source of bioactive secondary metabolites. Plant Genet Resour 3: 273-287.
- Yamamoto Y, Miura Y, Higuchi M, Kinoshita Y, Yoshimura I (1993) Using lichen tissue cultures in modem biology. The Bryologist96: 384-393.
- Yamamoto Y, Hara K, Kawakami H, Komine M(2015) Lichen substances and their biological activities. Recent Advances in LichenologySpringer, India,pp: 81-199.
- Performance Standards for Antimicrobial Disk Susceptibility Tests (2015)National Committee for Clinical Laboratory Standards M0-A12, Villanova, Pennsylvania, USA.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. American J Clin Pathol 45: 493-96.
- Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis 6:2.
- Neeraj V, Behera BC, Parizadeh H, Sharma BO (2011) Bactericidal Activity of Some Lichen Secondary Compounds of Cladonia ochrochlora, Parmotrema nilgherrensis and Parmotrema sancti-angelii. International Journal of Drug Development and Research 3: 3.
- Prabhu SS, Sudha SS (2015) Evaluation of the antibacterial properties of some Lichen species against human pathogens. Int J Adv Res BiolSci 2: 177-181.














