Keywords
Nutrition; Diabetes; Lipid profile; Immune response; Antidiabetic; Irvingia gabonesis seed
Introduction
It has been established that placebo and randomized trial treatment was an approach that led to the discoveries of some plants that are beneficial to human’s health. Such plants were used as food to increase perspiration, bowel movement, pain relief and healing. The use of phyto-medicine has been identified as a means of studying the potentiality of future medicines. Over production of free radicals and oxidative stress has been associated with the onset of diabetic complications [1]. Diabetes is a common metabolic disorder linked with diabetic ketoacidosis, hyperosmolar coma, cardiovascular problems, kidney failure, eye damage, non ketotic hyperosmolar coma and foot ulcers. The condition develops due to abnormalities in carbohydrate metabolism and insulin homeostasis, which give rise to high blood sugar level with symptoms such as elevated hunger and thirst, polyuria, glycosuria, and lethargy [2]. Several treatments have been made and success recorded, but the adverse effects associated with synthetic medicine are rather creating fears than remedy. For example, the application of a potent antidiabetic medicine called glucovance.
Glucovance is a combined therapy, made up of two andidiabetic medicines, which belong to biguanide (metformin hydrochloride) and sulphonylurea (glibenclamide). Metformin hydrochloride and glibenclamide have different mechanism and sites of actions. Though their actions are complement to each other. Metformin reduce hepatic glucose production, by increasing insulin sensitivity in the muscles (where lipid is concentrated), thereby delaying glucose absorption. On the other hand, glibenclamide stimulate the release of insulin by the pancreas. Therefore, this medicine is indicated for adults whose glyceamia level is stable and well control [3]. The adverse effect of this drug at slightest dereliction or contraindication are severly consequential. Its why the need for complementary and alternative phyto-therapy was investigated on lipid profile of diabetic wistar albino rats. Irvingia gabonenesis plant is indigenous to West Africa and known locally as bush mango, dikanut or African mango (Figure 1). The flesh of Irvingia is widely consumed [4]. Irvingia gabonenesis seeds are rich in oil [5], used for making paste (dika) (Figures 2 and 3). The shell is mixed with palm oil to treat diarrhoea and reduce breastfeeding duration, while the immature fruits are used to fasten ripening of unripe mature banana and fruits. The peels from the stem bark are consumed for hernias, yellow fever, and dysentery and to reducing the effects of poison [6]. The Mende tribe of Sierra Leone grind the bark into paste to apply on the skin for pain relief [7]. African bush mango fruit juice produces quality wine at 8% alcohol after 28 days fermentation. The much was comparable to the colour, flavour, sweetness and acceptability of a German reference wine [8]. The kernels of African mango are classified as oil seed. The fat extracted from the kernel is similar to margarine or cooking oil. Flour may also be produced from the kernel [9].

Figure 1: Irvingia gabonesis fruits.

Figure 2: Irvingia gabonesis seeds.
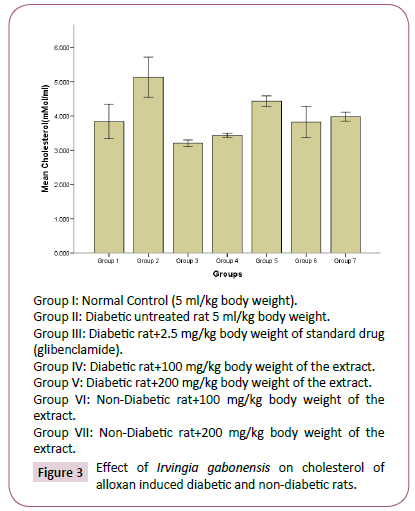
Figure 3: Effect of Irvingia gabonesis on cholesterol of alloxan induced diabetic and non-diabetic rats.
In diabetes, dikanut fibre finds its benefits in diabetic rats when it was administered in four weeks. The dikanut fibre supplement was effective than cellulose and membrane bound enzymes of the intestine and hepatic glycolytic enzymes, leading to reduced absorption of glucose [10]. This of course was similar in activity to metformine. A similar study was performed with streptozotocininduced diabetic rats, where dikanut fibre was introduced. Results showed reduction in plasma levels of glucose, cholesterol and triglyceride level [11]. In obesity, they were mechanisms against obesity with African bush mango supplementation; including inhibitory effects on glycerol-3-phosphate dehydrogenase was involved in converting glucose to stored fat [12]. Its beneficial effect on peroxisome proliferator-activated receptor (PPAR)- gamma involved adipogenesis and insulin sensitivity [13], similar to metformin action. Up regulation of adiponectin, which enhances insulin sensitivity and endothelial function, but decreased leptin expression by enhancing leptin sensitivity, inhibits food intake and stimulates thermogenesis. The antidiabetic effects of human type 2 diabetes and streptozotocin induced diabetic rats of the seeds extract have been reported [14]. The aqueous stem bark extract has demonstrated considerable anti-obesity and hypoglycaemic effect in normal rabbit [15]. African mango leaf and root extract have inhibitory activity against several bacteria and fungi strains [16]. It mechanism of action include membrane disruption by terpenoids and inactivation of microbial adhesion, enzymes and cell envelope transport protein by ellagic acidic compounds [17]. A methanol extract of African bush mango exhibited dose dependent inhibition of indomethacin-induced gastric ulceration in mice [18]. The administration of exogenous insulin treats type 1 and 2 diabetes. Current drugs used in diabetes management are grouped into three categories. The first group of drugs increase endogenous insulin availability. These include glibenclamide, the glinides insulin analogues, glucagon like peptide 1 (GLP-1) agonists and dipeptidyl peptidase-iv (DPP-iv) inhibitors. The first two members of these groups act on the sulfonylurea receptor in the pancreas to promote insulin secretion (GLP -1 agonists) and DPP-iv inhibitors as mention previously. The second group includes the thiazolidine-diones, which are agonists of the peroxisome proliferator-activated receptor gamma (PPAR?) and biguanide metformin. Biguanides derived from Galega officinalis is an excellent example of anti-diabetic drug developed from plants [19]. The third group comprises the α-glucosidase inhibitors such as acarbose which reduce the digestion of polysaccharides and bioavailability [20]. All the existing therapies however have limited efficacy, limited tolerability and/or significant mechanism based on their side effects [21]. This motivates the use of natural products for medicinal treatment of type 2 diabetes mellitus.
Materials and Methods
Plant material
The seeds of Irvingia gabonesis plant were bought from Ogige market in Nsukka local government area of Enugu state, Nigeria. Mr Alfred Ozioko of Bio-resources Development and Conservation Programme (BDCP), Nsukka Nigeria, identified and authenticated the fruits.
Animals
Thirty-five (35) adult Wistar albino rats of average weight 100-200 g and twenty-seven adult albino mice were used for this study. They were obtained from the animal house of the Department of Veterinary medicine, University of Nigeria, Nsukka. The animals were acclimatized for fourteen days with 12-hour light and dark cycle. They were placed on regular feed and water ad libitum.
Chemicals and reagents
The chemicals employed in the study were of analytical grade and were products from Sigma Aldrich, Germany and British Drug House, England.
Preparation of plant materials
The seeds of Irvingia gabonesis were oven dried at 50°C and pulverized into fine powder using a creston high speed grinder.
Extraction Procedure: A 558 g of the pulverized seeds of Irvingia gabonesis were macerated in a mixture chloroform- ethanol in a ratio 2:1 for 48 hours at room temperature. The macerate was filtered using Whatman No 4-filter paper and the filtrate shaken with 20% (v: v) of water to obtain two layers. The lower layer (chloroform layer) was separated from the upper layer (ethanol layer) using a separating funnel. Both layers were concentrated on a hot plate/magnetic stirrer at 40°C to 60°C. The extract was stored in an airtight plastic container in the refrigerator (4°C) and used for the study.
Acute toxicity test of ethanol extract of Irvingia gabonesis
The method of Rie et al. [22] was used for acute toxicity test of ethanol extract of Irvingia abonensis seeds.
Induction of Diabetes followed the method of Sheehan [23]. Determination of Blood Glucose followed the method described by Rotenstein [24]. Cholesterol Determination was done following the method of Lorke [25]. Triacylglycerol (TAG) was determined according to the method of Frode and Medeiros [26]. The highdensity lipoprotein (HDL) was determined according to method of Kunst et al. [27]. The low-density lipoprotein was determined according to the method of Kunst et al. [27]. Lipid peroxidation was estimated by measuring spectrophotometric technique as described by Abell et al. [28].
Experimental Design
Thirty-five (35) adult albino rats weighing between 100-180 mg/kg was grouped into 7 different cages of 5 rats per cage, acclimatized to laboratory standards for 9 days with free access to water and feed ad libitum. After acclimatization, their weights were measured and baseline glucose level was determined after overnight fasting prior to the induction of diabetes. Alloxan monohydrate was administered intraperitoneal with 200 mg/ kg body weight dissolved in ice cold normal saline. After two days the rats with blood glucose level greater than 16 mmol per liter were considered diabetic and used for the experiment. The treatment lasted for fourteen days (14) days, during which the blood glucose concentration of the rats was taken on days 0, 3, 7, 11 and 14. The route of administration was oral with the aid of intubation tube. The group and doses administered were summarized below:
Group 1: Normal control rats received 5 ml/kg body weight of normal saline.
Group 2: Positive control (diabetic untreated) rats received 5 ml/ kg below of normal saline.
Group 3: Diabetic rats plus 2.5 mg/kg body weight of glibenclamide.
Group 4: Diabetic rats plus 100 mg/kg body weight of ethanol extract.
Group 5: Diabetic rats plus 200 mg/kg body weight of ethanol extract.
Group 6: Non-diabetic rats plus 100 mg/kg body weight of ethanol extract.
Group 7: Non-diabetic rats plus 200 mg/kg body weight of ethanol extract.
Statistical analysis
Descriptive analyzed was done by using Statistical Product and Services Solution (SPSS) IBM version 21; results are expressed as mean ± Standard deviation. Significant was established by one-way analysis of variance ANOVA and acceptance level of significance was p<0.05.
Results and Discussion
During the investigation, normal rats (group 1) which were fed normal diets and water only showed fluctuations in their mean fasting blood glucose concentration from 73.80 ± 5.79 mg/dl on day 0, 117.00 ± 23.54 mg/dl on day 7 and decreased to 93.00 ± 12.4 mg/dl on the day 14. Group 2 fluctuate in mean blood glucose concentration from 318.60 ± 37.80 on day 0 to 315.03 ± 29.83 day 14. In group 3 treated with glibenclamide showed reduction in mean glucose level from day 0 to day 14. Group 4 rats treated with 100 mg of ethanol extract showed reduction in mean blood glucose concentration from day 0 to day 14 and group 5, had their fluctuation in mean blood glucose from day 0 to day 7 while reduction in mean blood glucose occur from day 7 to day 14, respectively. In group 6 and 7, the mean blood glucose concentration showed a fluctuation from day 0 to day 7 and decline on day 14. Therefore, Irvingia gabonesis seeds extracted with ethanol was presumed to have imparted, ameliorate diabetes mellitus and its associated complication by regulating blood glucose level. Treatment with standard drug (metformine) and ethanol extract in groups (3, 4 and 5) effectively lowered the blood glucose concentration from day 3 compared to diabetes untreated rat (group 2) (Figure 1). The hypoglycaemic effect was attributed to saponin, flavonoid and tannin function in lowering the plasma glucose concentration [29]. Alloxan is a hydrophilic chemical compound analogous to glucose. It is responsible for selective uptake and accumulation of pancreatic beta cell [26]. The extract may have exerted antihyperglycaemic activity by enhancing glucose uptake, stimulating insulin secretion from pancreatic β-cells and inhibiting pancreatic amylase [30] (Figures 4-7).
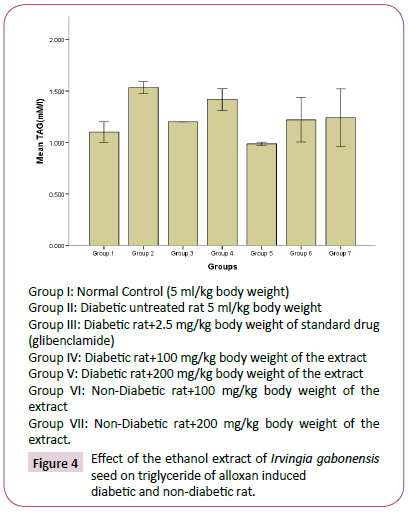
Figure 4: Effect of the ethanol extract of Irvingia gabonesis seed on triglyceride of alloxan induced diabetic and non-diabetic rat.
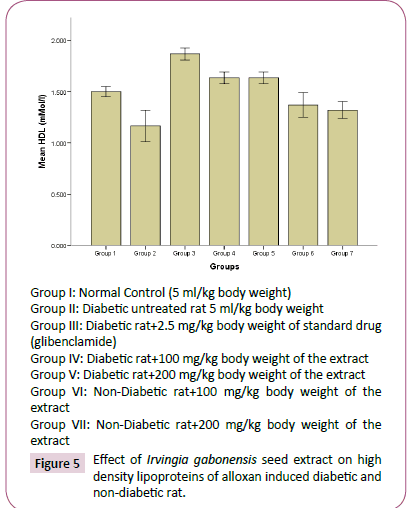
Figure 5: Effect of Irvingia gabonesis seed extract on high density lipoproteins of alloxan induced diabetic and non-diabetic rat.
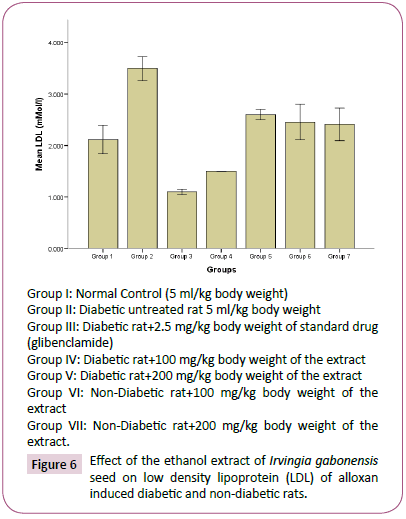
Figure 6: Effect of the ethanol extract of Irvingia gabonesis seed on low density lipoprotein (LDL) of alloxan induced diabetic and non-diabetic rats.
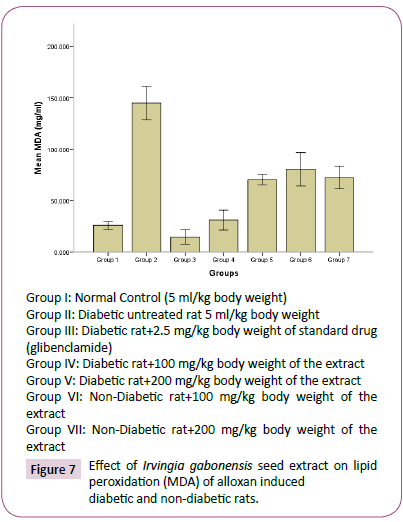
Figure 7: Effect of Irvingia gabonesis seed extract on lipid peroxidation (MDA) of alloxan induced diabetic and non-diabetic rats.
The high concentration of triglyceride, total cholesterol, low density lipoprotein and decrease in high density lipoprotein observed in diabetes untreated rats compared to normal rats group, agreed with reports of Reitman and Frankel [31], which suggests that an elevated glucose level will occur upon induction of diabetes, and this would give rise to corresponding increase in plasma lipids concentration. Hyperlipidaemia is a factor of cardiovascular diseases associated with diabetes mellitus. It is often characterized by elevated cholesterol, triglycerides, phospholipids and other lipoproteins [32]. The effect of Irvingia gabonesis seeds extract of on the plasma lipid profile of alloxan induced diabetic and non-diabetic rats was shown in Figures 3-7. The effect of Irvingia gabonesis seed extract on plasma total cholesterol of alloxan induced diabetic and non-diabetic rats is shown in Figure 4. There was a significant (p<0.05) increase in the plasma total cholesterol of group 2 (diabetes untreated) and group 5 (diabetic rats treated with 200 mg of ethanol extract) compared to normal control (group 1). A significant (p<0.05) reduction was observed in the plasma total cholesterol of group 3 (diabetic rat treated with glibenclamide) compared to normal and diabetes untreated rats. There was no significant (p>0.05) decrease in the total cholesterol of group 4 and 6 (diabetic and normoglycaemic rat treated with 100 mg of ethanol extract). However, a non-significant (p>0.05) increase was observed in the total cholesterol of group 7 (normoglycaemic rat treated with 200 mg of ethanol extract) compared to normal control rat. The effect of the ethanol extract of Irvingia gabonesis seed on triglyceride of alloxan induced diabetic and non-diabetic rat (Figure 5). The plasma triglyceride of diabetes untreated and diabetic rat treated with 100 mg of ethanol extract was significantly (p<0.05) high compared to normal control. A non-significant (p>0.05) increase in triglyceride of group 3, 6 and 7. However, triglyceride of group 5 shows a non-significant (p>0.05) decrease compared to normal rat. The effect of Irvingia gabonesis seed extract on high density lipoprotein (HDL) cholesterol of alloxan induced diabetic and nondiabetic rat (Figure 6). High density lipoprotein of group 2 shows a significant (p<0.05) increase compared to group 1 (normal control). A non-significant (p>0.05) decrease was observed in HDL cholesterol of group 6 and 7 (diabetic rat treated with 100/200 mg of ethanol (Extract) showed a non- significant (p>0.05) decrease compared to normal control. The effect of Irvingia gabonesis seed extract on low density lipoprotein (LDL) of alloxan induced diabetic and non-diabetic rats is shown in Figure 6. Low density lipoprotein cholesterol of group 2 (diabetes untreated) and group 5 shows significant (p<0.05) increase compared to group 1 (normal control). There was a significant (p<0.05) decrease in LDL cholesterol of group 3 and 4 compared to normal control and diabetes untreated. However, the LDL cholesterol of group 6 and 7 showed a non-significant (p>0.05) increase compared to normal control. The effect of the ethanol extract of Irvingia gabonesis seed on lipid peroxidation (MDA) of alloxan induced diabetic and non-diabetic rats (Figure 7). The malondialdehyde (MDA) of diabetes untreated group was significantly (p<0.05) high compared to normal control. There was a (p<0.05) increase in malondialdehyde of group 5, 6 and 7. However the MDA of group 3 shows a non-significant (p>0.05) decrease while Group 4 showed a non-significant (p>0.05) increase compared to group 1 (normal control). The effects of Irvingia gabonesis seed extract on the plasma hepatospecific markers of alloxan induced diabetic and non-diabetic rats.
Conclusion
The ethanol extract of Irvingia gabonesis seed is a potent antidiabetic agent due to it ameliorating effect on type 2 diabetes mellitus via reduction of total blood glucose concentration of alloxan induced diabetic rats. This provides claims that the plant (bark, leaves and seeds) was able to ameliorate abnormalities associated with Diabetes Mellitus.
Acknowledgement
All the authors contributed equally to this investigation.
20674
References
- Kong JM, Goh NK, Chia LS, Chia TF (2003) Recent advances in traditional plants drugs and orchids. Acta Pharmacologica Sinica 24: 7-21.
- Victor EO, Bayim PRB, Margaret OA (2014) Comparative effects of some medicinal plants: Anacardium occidentale, Eucalyptus globulus, Psidium guajava, and Xylopia aethiopica extracts in alloxan-induced diabetic male Wistar albino rats. Biochem Res Int Article ID 203051.
- Bonaventure CO, Theophine CO, Victor EO, Christiana NI, Edwin OA (2016) Comparative study of the antioxidant effects of metformin, glibenclamide and repaglinide in alloxan- induced diabetic rats. J Diabetes Hindawi Publishing Corporation Article ID 1635361.
- WHO (2013) In: World Health Organization (Ed) WHO Traditional Medicine Strategy 2014-2023. WHO Press, Geneva, Switzerland.
- Atangana AR, Tchoundjeu Z, Foldout JM, Assah E, Dumb M, et al. (2002) Domestication of Irvingia gabonesis: 1 phenotypic variation in fruit and kernels in two populations from Cameroun. Agroforestry System 53: 55-64.
- Hossain MS, Nahar N, Shoeb M, Hasan K, Sakeng S, et al. (2012) Hypoglycemic effect of Irvingia gabonesis (Aubry-Lacomate Ex. Ororke), Baill in type 2 diabetic long evans rats. Dhaka University J Pharma Sci11: 19-24.
- George IN, Zhao Y (2007) Pharmacological activity of 2, 3, 8 tri-o-methyl ellagic acid isolated from the stem bark of Irvingia gabonesis. Afri J Biotechno 6: 1910-1912.
- Okigbo RN, Mmeka EC (2006) An appraisal of phytomedicine in Africa. KMITL Science Technol J 6: 83-94.
- Leakey R (1999) Potential for novel food product from agroforestry trees: A review. Food Chemi 66: 1-14.
- Onyeike EN, Olungwe T, Uwakwe AA (1995) Effect of heat treatment and defatting on the proximate of some Nigerians local soup thickeners. Food Chem 53: 173-175.
- Leakey RRB, Greenwell P, Hall MN, Atangana AR, Usoro C, et al. (2005) Domestication of Irvingia gabonesis: 4. Tree to tree variation in food thickening properties and in fat and protein contents of dika nuts. Food Chem 90: 365-378.
- Atangana AR, Tchoundjeu Z, Fondon JM, Assah E Ndoumbe M, et al. (2005) Domestication of Irvingia gabonesis: Phenotypic variation in fruit and carnels in two populations from Cameroon. Agroforestry Sys 53: 255-264.
- Ngondi JL, Djiotsa EJ, Fossouo Z, Oben J (2006) Hypoglycaemic effect of the methanol extract of Irvingia gabonesis seeds on streptozotocin diabetic rats. Afr J Trad Comp Alt Med 3: 74-77.
- Dzeufiet DPD, Ngeutse DF, Dimo T, Tedong L, Ngueguism TF, et al. (2009) Hypoglycemic and hypolipidemic effects of Irvingia gabonesis (irvingiacaee) in diabetic rats. Pharmacology Online 2: 957-962.
- Oben JE, Ngondi JL, Blum K (2008) Inhibition of Irvingia gabonesis seed extract (OB131) on adipogenesis as mediated via down regulation of the PPAR gamma and leptin genes and up regulation of the adiponectin gene. Lipids Health Dis 7: 44-46.
- Ngondi JL, Etoundi BL, Nyangono CB, Mbofung CM, Oben JE (2009) A novel seed of the West African plant Irvingia gabonesis, significantly reduces body weight and improve metabolic Parameters in overweight humans in a randomized double-blind placebo controlled Investigation. Lipid Health Dis 8: 17-20.
- Ngondi JL, Oben JE, Minka SR (2005) The effect of Irvingia gabonesis seeds on body weight and blood lipids of obese subjects in Cameroun. Lipid Health Dis 4: 12-15.
- Omonkhua A, Onoagbe A (2012) Effects of long term administration of aqueous extract of Irvingia gabonesis bark on blood glucose and liver profile of normal rabbits. J Med Plants Res 6: 2581-2589
- Fadare DA Ajaiyeoba EO (2008) Phytochemical and anti-microbial activities of the wild mango-Irvingia gabonesis extracts and functions. Afri J Med Medical Sci 37: 119-124.
- Kuete V, Wabo GF, Ngameni B, Mbaveng AT, Metuno R, et al. (2007) Antimicrobial activity of the methanolic extract fractions and compounds from the stem bark of Irvingia gabonesis (Ixonanthaceae). J Ethnopharmac 114: 54-60.
- Raji Y, Ogunwande IA, Adesola JM, Bolarinwa AF (2001) Anti-diarrhegenic and anti-ulcer properties of Irvingia gabonesis in rats. Pharmaceutical Biol 39: 340-345
- Rie Y, Junichi Y, Naoko T (2005) Trend of antidiabetic drug. Basic and clinical review of biguanide. Clinical Endocrinol 53: 159-163.
- Sheehan MT (2003) Current therapeutic options in type 2 diabetes mellitus: A practical approach. Clinical Medicine Res 1: 189-200.
- Rotenstein LS, Kozak BM, Shwers JP, Yarchoan M, Close J, et al. (2012) The ideal diabetes therapy: What will it look like? How close are we? Clinical Diabetes 30: 44-53.
- Lorke D (1983) A new approach to practical acute toxicity testing. Archives of Toxicol 55: 275-287.
- Frode TS, Medeiros A (2008) Animal models to test drugs with potential antidiabetic activity. J Ethnopharmacol 115: 173-183.
- Kunst A, Draegor B, Ziegenhorn J (1984) Method of enzymatic analysis. Weinheim West Germany-Deerfields Beach, Florida, USA. pp. 178-185.
- Abell LL, Levy BB, Brodie BB, Kendall FE (1952) A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chemistry 195: 357-366.
- Otvos J (1999) Measurement of triglyceride-rich lipoproteins by nuclear magnetic resonance spectroscopy. Clinical Cardiol 22: 1121-1127.
- Kameswara R, Kesavulu B, Giri MM, Appa CH (1999) Antidiabetic and Hypolipidemic effects of Momordica cymbalaria Hook, fruit powder in alloxan diabetic rats. J Ethnopharmacol 67: 103-109.
- Reitman S, Frankel SA (1975) colorimetric method for the determination of serum glutamate, oxaloacetate and pyruvate transaminase. Am J Clinical Pathol 28: 56-63.
- Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, et al. (2004) American diabetes association. North American association for the study of obesity: American society for clinical nutrition. Weight management through lifestyle modification for the prevention of type 2 diabetes: Diabetes Care 27: 2067-2073.












