Ladu AI1,2*, Abba AM1,2, Bukar AA1,2, Abulfathi FA1,Kundili Y1, Talba HA1,Abba Kawu Y1, Tukur RA1 and Mohammad Y1
1Department of Haematology and Blood Transfusion, University of Maiduguri Teaching Hospital, Maiduguri, Borno State, Nigeria
2College of Medical Science, University of Maiduguri, Maiduguri, Borno State, Nigeria
*Corresponding Author:
Ladu-AI
Department of Haematology and Blood transfusion
University of Maiduguri Teaching Hospital
PMB-1069, Maiduguri, Borno State
Nigeria.
Tel: +234803772013
E-mail: adamaisahladu@gmail.com
Received date: May 14, 2018; Accepted date: May 31, 2018; Published date: June 05, 2018
Citation: Ladu AI, Abba AM, Bukar AA, Abulfathi FA, Kundili Y, et al. (2018) Assessment of Oxygen Saturation Using Pulse Oximetry in Patients with Steady State HbSS. Ann Clin Lab Res. Vol.6 No.2: 237. doi: 10.21767/2386-5180.100237
Keywords
Hypoxemia; Pulse oximetry; Sickle cell anaemia
Introduction
The lungs are a major site of involvement in patients with sickle cell disease (SCD), with both acute and chronic pulmonary complications commonly reported, and associated with increased mortality [1,2]. Sickle cell chronic lung disease presumably results from recurrent episodes of pulmonary infarction and infection. It is characterized by reduced lung luscency, abnormal pulmonary function, and in its severe form, pulmonary hypertension [1,3]. Moderate to severe pulmonary function impairment can results in hypoxemia, which may initiate or exacerbate vasculopathy [4]. There are reports of increased risk for central nervous system events with hypoxemia, whereas, higher oxygen saturations (SpO2) was associated with less frequent acute chest syndrome [5,6]. Many studies showed haemoglobin desaturation to be common in patient with sickle cell anaemia (SCA) even at steady state [7-9], and high prevalence of hypoxemia has been documented in children with SCA [10,11]. With improved care, the median survival of patients with SCA has risen, and therefore the prevalence of chronic organ diseases encountered in the adult population has also increased. In this study, SpO2 was assessed in adults with SCA using pulse oximetry, a widely utilized noninvasive technique. We sought to compare SpO2 of patients with HbSS in steady state and healthy individuals with HbAA genotype; and to determine the prevalence of of hypoxemia amongst HbSS patients during steady state.
Research Methodology
Ninety three patients with steady state HBSS were evaluated during routine outpatient visits at the Haematology unit of the University of Maiduguri Teaching Hospital, from January through June 2017. Forty-eight healthy age and sex matched HbAA participants were used as controls. Oxygen saturation was recorded using a finger pulse oximeter (Suaoki, Model FS20A). This uses the principle of spectrophotometery and photoelectric plethysmography in determining oxygen saturation [12]. The appropriate sensor was placed on the right or left index finger and the values recorded after at least 2 minutes of stable SpO2, determined as regular pulsatile photoplethysmography signal apparent on the visual display of the oximeter. All measurements were made while the patient was breathing room air. Low oxygen saturation was defined as SpO2 less than 96%, which predicts a PaO2 of less than 70 mm Hg based on a normal oxyhaemoglobin curve [10]. The data was analyzed using the statistical package for social sciences version 20.0 (SPSS Chicago III USA.). Normality of data was tested using Kolmogorov-Smirnov test, and continuous variables expressed using means (SD) or proportions and compared using Student’s t-test. A p value of <0.05 was considered significant for all statistical analysis.
Results
The HbSS patients were made up of 36 (38.7%) males and 57 (67.3%) females, while HbAA controls comprised of 24 (50%) males and 24 (50%) females; with a mean age of 23.11 (6.03) years for the HbSS patients and 25.8 (6.19) years for the HbAA controls (p=0.07). The pulse rates and SpO2 of HbSS patients and HbAA controls are illustrated in Table 1. The HbSS patients had a significantly lower mean SpO2 of 95.5% ( 4.1) compared to 99.06% ( 1.14) for the healthy HbAA group (p=0.0001). Male and female HbSS patients had a comparable SpO2 (95.8% vs. 96.1%, p=0.610). Similarly, mean SpO2 was similar in male and female HbAA group (p=0.258) (Table 2). The prevalence of hypoxemia among the HbSS patients was 30.25% compared to 2.7% amongst the HbAA (Table 3). The lowest SpO2 recorded among the HbSS patients was 88% (Figure 1) and 95% for the controls respectively (Figure 2).
| Parameters |
HbSS (N=93) |
HbAA (N=48) |
P value |
| Age |
23.11 ± 6.03 |
25.8 ± 6.19 |
0.07* |
| Pulse rate |
88.9 ± 12.2 |
82.1 ± 8.89 |
0.001* |
| SpO2 |
95.5 ± 4.1 |
99.06 ± 1.14 |
0.0001* |
*Significant result using Student T test
Table 1: Clinical parameters of HbSS patients and HbAA controls.
| Subjects |
Mean SpO2 |
Mean SpO2 |
P value * |
| |
Males |
Females |
|
| HbSS |
95.80% |
96.10% |
0.61 |
| HbAA |
98.90% |
99.20% |
0.258 |
*Significant result using Student T test
Table 2: Comparison of Mean SpO2 in HbSS and HbAA groups based on gender.
| |
Subjects (HbSS) |
|
|
Controls (HbAA) |
|
| SpO2 |
Number of participants (93) |
Cumulative % |
SpO2 |
Number of participants (48) |
Cumulative % |
| < 90% |
6 |
6.5 |
< 90% |
0 |
0 |
| 90-95% |
22 |
30.1 |
90-95% |
1 |
2.7 |
| 96-200% |
65 |
100 |
96-200% |
48 |
99.3 |
Table 3: Distribution of SpO2 in HbSS patients and HbAA control group.
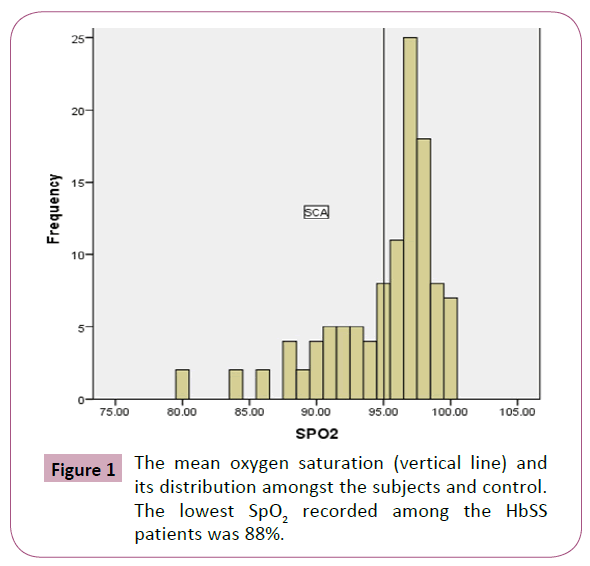
Figure 1: The mean oxygen saturation (vertical line) and its distribution amongst the subjects and control. The lowest SpO2 recorded among the HbSS patients was 88%.
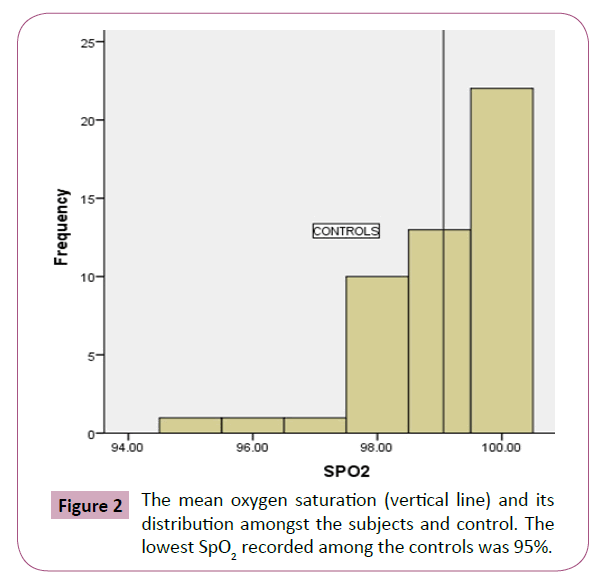
Figure 2: The mean oxygen saturation (vertical line) and its distribution amongst the subjects and control. The lowest SpO2 recorded among the controls was 95%.
Discussion
We found a prevalence of 30.5% for hypoxemia in steady state SCA patients using pulse oximetry, a finding similar to earlier reports [9,13]. Contrary to reports of lower SpO2 levels in male patients with steady state SCA [11,13], our male and female patients had comparable levels of SpO2. Hemoglobin desaturation in patients with steady state SCA has been attributed to several factors. Recurrent episodes of ACS could result in a sequalae of irreversible chronic lung disease, giving rise to defective oxygenation of blood even when in steady state [14]. In addition, the sickle cell hemoglobin S has an inherent property of causing a rightward shift of the oxy-hemoglobin dissociation curve in a bid to enhance oxygen delivery at the tissue level, a trait it shares with raised levels of 2,3-diphosphoglycerate characteristic of SCA [10,15]. In keeping with this, the oxygen dissociation curve of 32 clinically well HbSS patient, 12 HbSC patient and 10 normal control was compared at 70 mmHg, 80 mmHg and 90 mmHg of PO2. Despite having similar mean level of 2,3 DPG content, the oxygen dissociation curve of HbSS patient was more right shifted and significantly different when compared with those of the HbSC group (p=0.001). The authors inferred that HbS must have an effect on haemoglobin- oxygen affinity that is different from the effect of 2,3 DPG only. The HbSS patients had significantly lower mean resting oxygen saturation, than did patients with HbSC or the controls [5]. An earlier study had shown that oxygen affinity of HbSS blood is related to the intra cellular content of HbS and not 2,3 DPG [14]. All the subjects used for the index study were HbSS and may explain the low level of SpO2 even during steady state. However, hypoxemia is not a universal finding in patients with SCA and this raises some pertinent questions on the significance of hypoxemia in patients with steady state SCA and the best way to manage them, since treatment of apparent hypoxemia based on pulse oximetry in asymptomatic patients carries the deleterious consequences of suppressing erythropoiesis [16].
Pulse oximeter is often use as a surrogate for arterial measurement of oxygen saturation. In patients with normal oxygen dissociation curve, this reading is fairly accurate based on the assumption of a normal P50 of 26.5 mmHg [17]. There have been conflicting reports on the reliability of pulse oximeter in detecting hypoxemia in patients with SCA [8,14,18]. Pulse oximeter measures oxyhemoglobin as percentage of functional hemoglobin (oxyhemoglobin and deoxy-hemoglobin), whereas blood gas analysis for oxygen saturation measures it as a percentage of total hemoglobin including carboxy-hemoglobin and met-hemoglobin [14]. In patients with SCA patient undergoing hemolysis, the blood gas measurement may therefore underestimate oxyhemoglobin. Ratkoff et al. showed a wide variability in the oxygen dissociation curve of individuals with SCA and as a result, the SpO2 may be normal for a given partial pressure of oxygen [10]. In addition, the correlation between SpO2 measured by pulse oximetry and value obtained by calculation from blood gas co-oximetry has been shown to be excellent from these studies [10,14]. In low and middle income countries where the burden of SCA is very high, arterial blood gas testing is very scarce, hence, pulse oximetry is often relied upon to monitor oxygen saturation [11,19,20].
Conclusion
Chronic hypoxemia increases the risk of vasculopathy, pulmonary hypertension and cor pulmonale, a life-threatening complication in young adults with SCA [3,4,16]. Hemolysis has also been implicated in the pathogenesis of PH [16]. The levels of hemoglobin and reticulocytes, both potent markers of hemolysis, had been shown to be associated with hypoxemia in patients with HbSS [13,20]. Hypoxemia is associated with increased risk of central nervous system (CNS) events in patients with SCA [21,22]. This finding formed the premise on which screening and appropriate management of nocturnal hypoxemia was suggested as a safe and effective alternative to prophylactic blood transfusion for primary prevention of CNS events in patients with SCA [5]. Similarly, another study revealed that the odds of having stroke was 1.32 for each 1% decrease in SpO2, with a further drop in the SpO2 value during an impending stroke [8]. However, even with a normal daytime SpO2, hypoxemia remains a concern in patients with SCA, as it does not exclude nocturnal hypoxia which has been associated with neurological complications [7]. This makes the detection of hypoxemia an important aspect of the management of patients with SCA.
Future Considerations
The prevalence of hypoxemia in steady state HbSS patients is high. This finding underscores the importance of monitoring HbSS patients for prompt detection of hypoxemia, and to institute therapy where necessary. However, despite the association of low oxygen saturation with CNS event and other serious complications, there is still no agreed treatment modality. Previous reports have shown a strong positive correlation between high HbF and increased SpO2 [23,24]. Pashankar et al. reported on the improvement of oxygen saturation following treatment with hydroxyurea (HU). Hydroxyurea increases HbF level (which has a higher affinity for oxygen than HbS or HbA), and shifts the oxygen dissociation curve to the left which is associated with a high oxygen saturation. There results showed that after a 6 month of treatment, HU significantly increased oxygen saturation from a baseline of 95.15% to 98.4%, with the response sustained at 12 months post-treatment. This finding could pave way for the use of HU in SCA patients with hypoxemia; however, larger academic studies are needed to replicate this report.
Recommendations
In our study, HbSS patients had a suboptimal mean steady state SpO2 of 95.7%, with the lowest SpO2 of 88% despite having no evidence of respiratory distress. This underscores the importance of measuring SpO2 during routine evaluation of SCA, as this will provide baseline data against which comparison can be made during acute illnesses, especially when acute chest syndrome is suspected. Baseline SpO2 data may also be useful in prognostication, as well as inform the basis for further evaluation of asymptomatic pulmonary dysfunction e.g. pulmonary hypertension.
Conflict of Interest
None.
22802
References
- Knight J, Murphy TM, Browning I (1999) The lung in sickle cell disease. Pediatr Pulmonol 28: 205–216.
- Powars DA, Weidman JA, Odom-Maryon TA, Niland JC, Johnson CA (1988) Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine 67(1): 66-76.
- Siddiqui AK, Ahmed S (2003) Pulmonary manifestations of sickle cell disease. Postgrad Med J 79: 384–390.
- Caboot JB, Allen JL (2014) Hypoxemia in sickle cell disease: significance and management. Paediatr Respir Rev 15(1): 17-23.
- Kirkham FJ, Hewwes DKM, Prengler M, Wade A, Lane R, et al. (2001) Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. The Lancet 357(9269): 1656-2659.
- Hargrave DR, Wade A, Evans JPM, Hewes DKM, Kirkham FJ (2003) Nocturnal oxygen saturation and painful sickle cell crises in children. Blood 101(3): 846–848.
- Halphen I, Elie C, Brousse V, Le Bourgeois M, Allali S, et al. (2014) Severe Nocturnal and Postexercise Hypoxia in Children and Adolescents with Sickle Cell Disease. PLoS ONE 9(5): e97462.
- Quinn CT, Sargent JW (2008) Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. Br J Haematol 140(3): 336–339.
- Homi J, Levee L, Higgs D, Thomas P, Serjeant G (1997) Pulse oximetry in a cohort study of sickle cell disease. Clin lab haematol 19(1):17-22.
- Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K (1993) Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood 81: 3422–3427.
- Chinawa JM, Ubesie AC, Chukwu BF, Ikefuna AN, Emodi IJ (2013) Prevalence of hypoxemia among children with sickle cell anemia during steady state and crises: A cross-sectional study. Nigerian Journal of Clinical Practice 6(1): 2.
- Bowes WA, Corke BC, Hulka J (1989) Pulse oximetry: A review of the theory, accuracy and clinical applications. Obstet Gynecol 74: 541.
- Quinn CT, Ahmad N (2005) Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol 131(1): 129–134.
- Ortiz FO, Aldrich TK, Nagel RL, Benjamin LJ (2012) Accuracy of pulse oximetry in sickle cell disease. Am J Respir Crit Care Med 159: 447–451.
- Seakins M, Bigs WN, Milner PF, Bertles JF (1973) Erythrocyte Hb-S concentration: An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest 52: 422.
- Blaisdell CJ, Goodman S, Clark K (2000) Pulse oximetry is a poor predictor of hypoxemia in stable children with sickle cell disease. Arch Pediatr Adolesc Med 154(9): 900-903.
- Severinghaus JW, Kelleher JF (1992) Recent developments in pulse oximetry. Anesthesiology 76:1018–1038.
- Zheng S, Ruiz G, Chan F, Chakravorty S, Bossley C, et al. (2016) Poor agreement between haemoglobin oxygen saturation measured by pulse oximetry and arterialized earlobe blood gas in ambulatory paediatric sickle cell patients. European respiratory Journal 48: PA1217.
- Ogah AO, Surat A, Okoruwa AG, Ezeonwumelu JOC, Okolo SN (2012) Oxyhemoglobin saturation in sickle cell anaemic children (steady state) using pulse oxymetry in Jos University Teaching Hospital, Jos, Nigeria. Asian J. Med. Sci 4(5): 161-165.
- Saad AA, Ibrahim SH, Salih KMA (2016) Oxyhemoglobin saturation in children with sickle cell anemia during steady state and crises using pulse oximetry in Omdurman pediatric hospital- Omdurman, Sudan. Indian Journal of Medical Research and Pharmaceutical Sciences 3(11): 2-6.
- Sharon E, Cox SE, Makani J, Newton CR, Prentice AM, et al. (2013) Hematological and genetic predictors of daytime hemoglobin saturation in Tanzanian children with and without Sickle cell anemia. ISRN Hematology.
- Jamie M, Kawadler JM, Fenella J, Kirkham FJ, Clayden JD, et al. ( 2015) White matter damage relates to oxygen saturation in children with sickle cell anemia without silent cerebral infarcts. Stroke 46:1793-1799.
- Nkya S, Mgaya J, Urio F, Makubi A, Thein SL, et al. (2017) Fetal hemoglobin is associated with peripheral oxygen saturation in sickle cell disease in Tanzania. EBioMedicine 23: 146–149.
- Pashankar FD, Manwani D, Lee MT, Green NS (2015) Hydoxyurea improves oxygen saturation in children with sickle cell disease. Journal of Pediatric Hematology/Oncology 37(3): 242-243.








