Keywords
|
| Mitochondrial bioenergetics, Cancer-related fatigue, Prostate cancer, Radiation therapy |
Introduction
|
| Prostate cancer is a highly prevalent carcinoma and the second leading cause of cancer mortality in the United States [1]. The American Cancer Society estimates that 2015 will see 220,800 new diagnoses of this disease and 27,540 deaths [1]. Localized external beam radiation therapy using an intensity-modulated radiation technique, is a standard treatment option for nonmetastatic prostate cancer [2]. Although localized radiation therapy (XRT) has increased survival rates for men with this disease, fatigue is highly prevalent during and at the completion of treatment [3] and causes long-lasting distress even in diseasefree stages [4-6]. |
| Of all disease and treatment-related symptoms in cancer, fatigue is one of the most burdensome with the greatest adverse effect on quality of life, but arguably the least understood [7,8]. Fatigue experienced by prostate cancer patients has been noted to increase significantly in severity during the course of XRT, remaining elevated during survivorship, after treatment is completed [9,10]. The prevalence and severity of fatigue differs slightly in cancer patients receiving different treatments. Fatigue severity also differs between prostate cancer patients with XRT and without XRT [8]. While there have been a limited number of interventions suggested to address fatigue, the only one that has an adequate evidence based to date is exercise [11]. Thus, healthcare professionals attempting to assist patients in addressing this critical source of distress have few strategies that can be helpful. The use of modafinil, management of activity and psychoeducational interventions are the only tools currently recommended [12]. However, early non-randomized trials of nutriceutical supplements, such as levocarnitine or vitamins offer an intriguing possible avenue for nursing intervention. Nevertheless, there remains a critical need to develop a better understanding of biologic mechanisms of fatigue before we can move forward in testing interventions. |
| Localized radiation-induced damage is associated with many adverse effects including fatigue [13]. Although many mechanisms have been proposed for cancer-related fatigue [14- 18], the cause remains elusive. Additionally, early biomarkers prognostic for radiation-induced fatigue have not been identified. The physiological mechanisms behind fatigue and its increased severity during localized XRT remain unknown. Deficiency of adenosine triphosphate (ATP) has been proposed as the basis of cancer-related fatigue [16,19], but the mechanism has not been explored. |
| More than 90% of ATP is generated by mitochondria via oxidative phosphorylation (OXPHOS) [20]. Mitochondrial respiratory chain is very important in maintaining effective ATP levels [21]. Mitochondrial dysfunction is involved in all clinical conditions including fatigue which are associated with the deficient energy metabolism of oxidative phosphorylation [22]. While mitochondrial dysfunction has been implicated in a variety of clinical fatigue states, the physiological pathways and pathophysiological mechanisms are complicated and remain unclear. |
| This research proposal is designed to determine the association between mitochondrial bioenergetics and fatigue symptom in non-metastatic prostate cancer patients receiving XRT. The specific aims include |
| 1.Determine changes in mitochondrial bioenergetics profile in lymphocytes from prostate cancer patients at baseline, midpoint, and endpoint of the XRT |
| 2.Quantify fatigue symptom in prostate cancer patients at baseline, midpoint, and endpoint of the XRT and |
| 3.Determine the association between mitochondrial bioenergetics and fatigue symptom in prostate cancer patients at baseline, midpoint, and endpoint of the XRT. |
Background
|
| Fatigue is one of the debilitating symptoms most often reported to nurses by cancer patients receiving XRT [23]. Cancer-related fatigue is described as pervasive, a whole body excessive tiredness that is unrelated to activity or exertion, and not relieved by rest or sleep [24]. Cancer fatigue negatively impacts health outcomes leading to increased depression, impaired cognitive function, increased sleep disturbance, decreased physical activity and decreased health-related quality of life [25-28]. Multidimensional causes and mechanisms of cancer-related fatigue remain unclear, and early biomarkers prognostic for radiation-induced fatigue have not been identified. |
| Cancer fatigue is arguably the least understood cancer-related symptom [29]. Moreover, there is no optimal pharmacologic therapy for fatigue. The National Comprehensive Cancer Network (NCCN) Practice Guidelines in Oncology for cancerrelated fatigue currently recommends 5 non-pharmacological interventions which are activity enhancement, psychosocial improvement, attention-restoring therapy, nutrition, and sleep [12]. For pharmacologic interventions, the NCCN guidelines recommend that after ruling out other causes of fatigue the use of psychostimulants should be considered. Specifically, methylphenidate has been recommended, but they are conflicting results in improving fatigue in two small, randomized, clinical trials [12,30]. With limited available options for nurses to address cancer-related fatigue, novel strategies are needed to identify effective interventions for cancer-related fatigue. |
| The interactions of several mechanisms have been proposed to influence the individual fatigue experience, including genetic factors, energy expenditure, metabolism, aerobic capacity, and the patients’ immune response to inflammation [29,31]. Research has shown the genetic factors influence cellular response associated with XRT [32]. Moreover, changes in gene expression in peripheral leukocytes have been seen in fatigue cancer patients receiving XRT [18,33]. Reactive oxygen species (ROS) are considered one of the major direct causes of ionizing radiation-induced damage [34], resulting in a number of adverse effects including fatigue [35]. |
| It is known that radiation-induced damage alters mitochondrial metabolism, inhibits the mitochondrial respiratory chain, and forms highly reactive peroxynitrite (ONO2 -) [36]. Once mitochondrial proteins are damaged, the affinity of substrates or enzymes is decreased resulting in mitochondrial dysfunction [20]. However, these studies did not identify the mechanism of the inhibiting mitochondrial respiratory chain related to mitochondrial dysfunction after radiation. Identification of the mitochondrial bioenergetics mechanism leading to radiationinduced fatigue is needed in order to develop appropriately targeted therapies. |
| The mitochondrial respiratory chain is essential to produce and to maintain effective cell content of ATP [20,21]. A reduction in the capacity of neutrophils’ mitochondria to utilize oxygen and synthesize ATP has been associated with chronic fatigue syndrome [37]. Our previous study has shown that changes in mitochondrial-related gene expression (e.g. down-regulation of BCS1L and up-regulation of SLC25A37) in lymphocytes were associated with fatigue symptoms experienced by men with nonmetastatic prostate cancer during XRT [9,38]. Decreased BCS1L protein has been shown to lead to decreased incorporation of the Rieske iron-sulfur protein into complex III and decreased activity of complex III [39]. A defect in complex III will impair ATP production through a decrease in oxidative phosphorylation [40,41]. Additionally, decreased complex III activity is associated with increased superoxide (O2 -) production and dismutation to hydrogen peroxide (H2O2) [42,43]. Furthermore, up-regulation of SLC25A37 increases the mitochondrial inner membrane mitoferrin-1 protein [44]. Increased mitoferrin-1 protein leads to increased iron uptake into mitochondria and promotes heme synthesis [45], and this increased matrix free iron potentially can increase hydroxyl radical formation from hydrogen peroxide [46]. |
Preliminary work
|
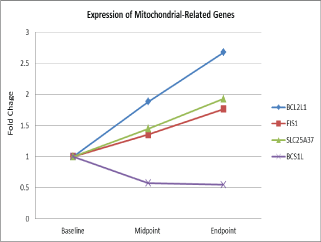
| Eleven mitochondrial related genes were differentially expressed in 25 men with non-metastatic prostate cancer during external beam radiation therapy over time [44]. Figure 1 depicts that one down-regulated gene, BC1 (ubiquinol-cytochrome c reductase) synthesis-like (BCS1L) and 3 up-regulated genes (BCL2-like1, (BCL2L1), solute carrier family 25, member 37(SLC25A37), and fission 1 homolog (S. cerevisiae) (FIS1) were differentiated expressed during XRT in fatigue men with prostate cancer. These genes were significantly associated with fatigue scores (β= 1.3- 2.4, p= 0.001) at the completion of radiotherapy compared to the baseline [38]. |
| Differential expression of two genes inside the mitochondria involved in critical mitochondrial complexes: BCS1L (β=1.30), SLC25A37 (β=-2.44) and two genes on the outer mitochondrial membrane vital for mitochondrial integrity: BCL2L1 (β=-1.68), and FIS1 (β=-2.35) were significantly associated with changes in fatigue scores of study subjects during EBRT [38]. Additionally, altered gene expressions were accompanied by changes of protein concentrations in peripheral cell lysates [9,38]. |
| The human BCS1L gene encodes a member of the AAA family of ATPases, involved in the essential role of assembling complex III of the mitochondrial respiratory chain [40]. The mitochondrial respiratory chain is essential for maintaining effective ATP levels [21]. Mitochondrial oxidative phosphorylation enzymes, proteins and lipids are vulnerable to free radicals [47]. Decreased BCS1L protein has been associated with a deficient incorporation of the Rieske iron sulphur protein into complex III, resulting in decreased complex III activity. A defect in complex III leads to a functional deficit in the respiratory chain and impairs ATP production [48]. |
| SLC25A37 is a solute carrier localized in the mitochondrial inner membrane that serves as the principal iron importer [27]. SLC25A37 provides a critical role of iron-consuming processes including heme synthesis and Fe-S cluster synthesis in mitochondria [45,49]. Over expression of SLC25A37 and increased mitoferron-1 protein lead to increased iron uptake into mitochondria and promotes heme synthesis [45], and this increased matrix free iron potentially can increase hydroxyl radical formation from hydrogen peroxide [46]. Moreover, iron overload affects the mitochondrial calcium uniporter, slow calcium uptakes, and results in mitochondrial dysfunction [50], which may intensify fatigue experienced by men treated with XRT. |
| BCL2L1encodes proteins that belong to the BCL-2 family and are generally located on the mitochondrial outer membrane (MOM), which regulates the opening of the MOM’s voltage-dependent anion channel (VDAC) [51]. VDAC regulates the mitochondrial membrane potential by binding with BCL-2 family of proteins, therefore controlling the production of ROS and release of cytochrome c, both of which inducers of cellular apoptosis [52]. Overexpression of BCL2L1 blocks the programmed cell death through the leakage of MOM and the release of cytochrome c to initiate the cell intrinsic death pathway [53]. The tail-anchored outer membrane protein, Fis-1 is distributed on the mitochondrial surface serving as a ratelimiting fission factor. Inhibition of FIS1 has been shown to lead to an accumulation of damaged mitochondrial material, decreased metabolic function, and reduction in insulin secretion [54]. |
| In Summary, this preliminary work has established that fatigue is associated with mitochondrial-related genes, which may in turn be associated with mitochondrial biogenesis and bioenergetics in cancer patients receiving radiation therapy. What we do not know is if radiation induces changes in mitochondrial bioenergetics causing ATP depletion and contributing to fatigue. Therefore, BCS1L and SLC25A37were selected to test in the hypothesis of the mechanism causing radiation-induced fatigue, because they plan important roles in the mitochondrial respiratory chain where it produces and maintains effective ATP content. |
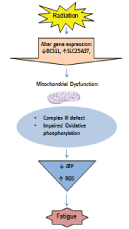
| In Figure 2, we proposed a physiological model of radiationassociated fatigue based on our preliminary findings. We hypothesized radiation will cause genetic instability and cellular damage, trigger a defect in mitochondrial OXPHOS, and cause ATP depletion and ROS production, resulting in debilitating fatigue. |
Materials and Methods
|
|
Study design
|
| This prospective, hypothesis-testing project will use a matched case-control, repeated measures design. Two groups of subjects will be recruited in this study: prostate cancer patients with localized radiation therapy (XRT) and prostate cancer patients without any treatment, active surveillance (AS). To determine if mitochondrial bioenergetics profile and fatigue symptom observed during XRT are associated with radiation therapy, we will use age-, race-, and clinical stage- matched prostate cancer patients with AS as the control for the comparison of fatigue and mitochondrial bioenergetics at baseline. Furthermore, changes in mitochondrial bioenergetics profile and fatigue, and association between changes in bioenergetics and fatigue will be determined in men receiving XRT at midpoint and endpoint of XRT, comparing to their baseline data. |
|
Sample and setting
|
| The study sample will be drawn from a population of localized prostate cancer patients scheduled for XRT. The study will be introduced to collaborating clinicians during one-on-one or team meetings at the National Cancer Institute designed Comprehensive Cancer Center in Northern Ohio. Patients will be referred by the collaborating clinicians to the study investigator for screening. The sample will consist of 25 individuals who meet the study criteria and provide consent. |
|
Inclusion criteria are
|
| 1.Clinically localized prostate cancer. 2. Scheduled to receive localized radiation therapy (e.g. external beam radiation therapy either by 3D conformal or IMRT techniques). 3. Able to provide written informed consent. 4. ≥18 years of age. |
|
Exclusion criteria are
|
| any one of the following: 1. Progressive or unstable disease other than cancer of any body system causing clinically significant fatigue (e.g., class IV congestive heart failure, endstage renal disease, stage IV chronic obstructive pulmonary disease) including patients with systemic infections (e.g., human immunodeficiency virus [HIV], active hepatitis); documented recent (<3 years) history of major depression, bipolar disease, psychosis, or alcohol/drug dependence/ abuse; uncorrected hypothyroidism, untreated anemia; and those with chronic inflammatory disease (e.g. rheumatoid arthritis, systemic lupus erythematosus). 2. Patients regularly taking antipsychotics and anticonvulsants, since these medications cause significant fatigue. 3. Patients who have second malignancies or those receiving chemotherapy with their EBRT. 4. Mitochondrial disease. 5. Taking medication for fatigue (e.g. methylphenidate, moldafinil). |
| This project intends to enroll 25 research participants from each group over a one year period. In our preliminary work [55], the BCS1L average ± standard deviation expression values at baseline, midpoint, and endpoint were 6.60 ± 1.22, 7.76 ± 1.64, and 7.64 ± 1.29, respectively, in prostate cancer patients with EBRT. Given this, for our proposed study, a sample size of 22 will achieve at least 80% power to detect a mean of paired differences of 0.58 (half of 1.15) with an estimated standard deviation of differences of 0.93 using a one-sided paired t-test and level of significance 0.05. |
Study measures
|
| Fatigue is essentially a subjective experience [56], and measurement of fatigue is a challenging process [57]. Subjective experience of fatigue is of equal importance to objective measurement of fatigue, because the symptom of fatigue is a complex phenomenon with multiple dimensions [58,59]. Therefore, for this study, the revised Piper Fatigue Scale and Patient Report Outcomes Measurement for Fatigue have been selected to assess the subjective dimensions of fatigue experienced by men treated for prostate. |
|
Fatigue
|
| Fatigue will be evaluated by validated questionnaires- The revised Piper Fatigue Scale (r-PFS) and Patient Reported Outcomes Measurement Information System for Fatigue (PROMIS-F). The r-PFS is a 22-item paper/pencil questionnaire that measures 4 fatigue dimensions including behavioral/severity, sensory, cognitive/mood, and affective. The r-PFS shows good reliability and validity with internal consistency ranging from 0.7-0.9 across 4 fatigue dimensions from cancer patients undergoing XRT [58]. It can be completed in 10 minutes. The PROMIS-F was developed from more than 1000 datasets from multiple disease populations including cancer. Initial psychometric properties showed internal consistency reliability coefficient of 0.80 [60]. It consists of 7-item questionnaire for fatigue and takes about 2 minutes to complete the questionnaire. |
|
Depression
|
| Hamilton Depression Rating Scale (HAM-D) will be use to assess depression symptom. The HAM-D is a 21-item with good internal reliability (α=0.8-0.9), completed by study staff through subject interview and screening process. Score can range from 0 to 78; higher scores (>17) indicate higher symptoms of depression [61]. It takes approximately 15 minutes to complete. |
|
Physical activity
|
| The International Physical Activity Questionnaire (IPAQ) will be used to evaluate physical activity levels for each participant. The IPAQ is a well validated, 7-item self-report questionnaire, and ask subjects to recall the amount of physical activity undertaken for the pass 7 days [62]. It takes approximately 5 minutes to complete. |
Mitochondrial Bioenergetics Profile Includes
|
| 1. Mitochondrial OXPHOS, |
| 2. The electronic transport chain (ETC) complexes activity, and |
| 3. ATP production and ROS generation |
|
Mitochondrial oxidative phosphorylation rate
|
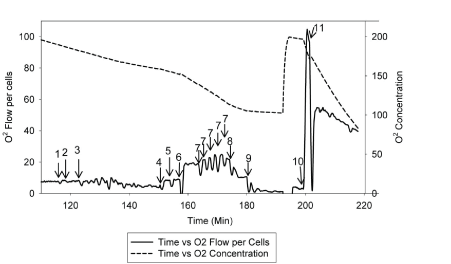
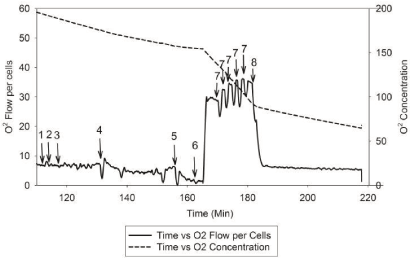
| The rate and changes of mitochondrial oxidative phosphorylation will be measured using patients’ lymphocytes. Dr. Hoppel and his research team have developed a new approach to measure integrate mitochondrial OXPHOS from human fibroblasts [63]. The standard laboratory procedure has been optimized and tailored to use of human lymphocytes in order to test the proposed hypothesis. Figure 3 and Figure 4 describe the substrate-inhibitors tracing for protocol 1 and 2 in human lymphocytes. |
| Two milliliter of respiration buffer with intact lymphocytes will be injected to two chambers of the Oroboros-Oxygraph-2k (O2K) system. Data will be normalized to cell number and protein concentration, as well as citrate synthase activity, which will be measured in each experiment as a marker of the amount of mitochondria per chamber. As described [63], mitochondrial OXPHOS will be measured starting with intact cellular respiration after air calibration of the O2K system [64]. The purpose of protocol 1 (Figure 3) is to measure the respiration rate of complexes I, II, and IV. The respiration rate does not change by adding endogenous substrates (malate and pyruvate) because the transporters are not present in the intact cells. To be able to have access to the mitochondria, we will use digitonin to permeabilize the plasma membrane, and a decreased respiration rate will be observed. When the rate reaches its nadir, adenosine diphosphate (ADP) will be added to obtain the state 3-ADP stimulated respiration rate. Next, we will add glutamate to provide additional substrate to make NADH for complex I, and then succinate will be injected as a complex II substrate to reduce coenzyme Q (Co Q) and measure complexes I and II substrate oxidation, and assess the availability of Co Q. After that, an uncoupler, carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP) will be titrated to reach the maximal oxidation. After that, rotenone will be added to obtain the rate of complex II because complex I is been inhibited. Then antimycin A will be added as a complex III inhibitor to obtain the residual rate, considering as nonmitochondrial oxidation. At the end, to obtain the rate of complex IV, we will add tetramethyl-p-phenylenediamine (TMPD) and ascorbate to reduce cytochrome C for complex IV as uncoupled, and sodium azide will be injected to inhibit complex IV. |
| Protocol 2 (Figure 4) is to measure fatty acid oxidation and complex III respiration rate. The respiration rate does not change by adding malate and followed by injecting palmitoylcarnitine to obtain fatty acid oxidation. Same as protocol 1, we will add digitonin to permeabilize the plasma membrane and the oxygen consumption rate will be gradually decreased over 15 minutes. When the rate reaches its nadir, ADP will be added to obtain the state 3-ADP stimulated respiration rate. Next, rotenone will be added to inhibit complex I, and then, a reduced analog of coenzyme Q, duroquinol, will be added as the complex III substrate to yield the respiration of complex III. After that, multiple titrations of FCCP will be performed to obtain the maximum oxidative capacity of complex III. Lastly, antimycin A will be added to inhibit complex III and protocol 2 is completed. |
|
The ETC complexes activity
|
| The activity of four complexes (I, II, III, IV) in the mitochondrial ETC will be measured using isolated lymphocytes in a spectrophotometer [65]. First, rotenone-sensitive NADH cytochrome c reductase will be used to measure the linked activity of complex I and III. Second, antimycin A-sensitive succinate cytochrome c reductase, will be used to measure the linked activity of complex II and III. Third, antimycin A-sensitive decylubiquinol cytochrome c reductase (complex III), will be used to measure the reduction of cytochrome c coupled to the oxidation of decylubiquinol to decylubiquinone. Complex III is inhibited by antimycin A and the assay will be measured as an antimycin A-sensitive component. Lastly, cytochrome c oxidase (complex IV) will be measured and it is the terminal component of the ETC and oxidizes reduced cytochrome c and converts oxygen to water. |
|
ATP production and ROS generation
|
| The total cellular ATP amounts will be determined using a convenient bioluminescence assay to quantify ATP with recombinant firefly luciferase and its substrate. A standard curve for a series of ATP concentrations will be generated for each assay and the ATP amount will be calculated compared to the standard curve. The rate of H2O2 production will represent the ROS production, and will determine using the oxidation of the fluorogenic indicator amplex red in the presence of horseradish peroxidase [66]. The concentrations of horseradish peroxidase and amplex red will be measured using a microplate reader through fluorescence. |
Data Collection and Procedure
|
| The study duration will be approximately 18 months. Localized radiation therapy for prostate cancer is usually administered 5 days a week for 7-9 weeks, depending on the type of treatment delivery and dose used. In this study, three time points (baseline, midpoint, end point) of data collection have been chosen to represent different phases of radiation therapy process and based on the peak of self-reported fatigue from prostate cancer patients treated with XRT. The healthy individual group will be asked to provide data at one time. Before starting the study, participants will be screened for eligibility by the investigator and then scheduled for data collection on their convenience. All tests and study visits will be conducted at the University Hospitals Seidman Cancer Center. Blood draws and self-administered questionnaires will be coordinated with all clinical care procedures related to the participant’s XRT schedule so that unnecessary duplication of tests and inconvenience can be avoided. |
| One 45 ml peripheral blood sample (3 tablespoons) will be collected from each patient at baseline, midpoint, and endpoint of the XRT. The tubes then will be transported to the mitochondrial research laboratory. Lymphocytes will be isolated from blood anticoagulated with EDTA tubes immediately once the tubes are delivered to the laboratory for processing, as explained in previous section. |
Data Analysis
|
| The study is designed to examine the association between mitochondrial bioenergetics and fatigue in prostate cancer patients receiving XRT over a period of time. Descriptive statistics (means ± standard deviation) will be calculated to describe mitochondrial bioenergetics profiles at each time point and the incidence and severity of fatigue at each time point. We will use twosample t-tests and analysis of covariance, adjusting for possible confounders such as age, race, and clinical stage to compare difference in fatigue and bioenergetics profile at baseline between XRT and AS group. To present the changes of fatigue score and mitochondrial bioenergetics profiles (oxidative phosphorylation, ETC complexes, ATP production, and ROS generation) before, at midpoint, and at endpoint of XRT, we will perform paired t-tests between baseline and midpoint, baseline and endpoint, and midpoint and endpoint. |
| Linear mixed model will be used to determine the associations between mitochondrial bioenergetics and fatigue in prostate cancer patients at baseline, midpoint, and endpoint of the XRT. The intercept and slope of the individual growth curve for fatigue scores and mitochondrial bioenergetics will be estimated using mixed model analysis. In the model, we will use time variables in terms of days during EBRT and a simple linear relationship will be assumed in the time variable. The intercepts and slopes of the outcome variables (changes in fatigue scores) and the predictors (changes in mitochondrial bioenergetics) for each participating individual will be estimated in the mixed model. Based on the variances and co-variances of the random effects, we will compute the correlations between fatigue and the other variables under consideration. If the measured continuous variables are not normally distributed, appropriate transformations (e.g. log, square root, etc) will be applied to meet the assumptions of statistical tests. All statistical analyses will be performed using Stata 11.0 software [67]. |
Discussion
|
| Through this study, we propose a novel mechanism of mitochondrial bioenergetics for cancer-related fatigue based on a molecular-genetic approach. The proposed physiological mechanism of cancer-related fatigue is linked to ATP depletion and impairment of mitochondrial bioenergetics, triggered by radiation-induced genetic instability and cellular damage. Currently, there are no evidence-based interventions, such as optimal pharmacologic therapy, nutritional supplements, or dietary interventions, for cancer-related fatigue. |
| This will be the first study to determine the role of mitochondrial metabolism, specifically bioenergetics function, in the development of debilitating radiation-induced fatigue. The study represents an opportunity for new insights into a moleculargenetic and mitochondrial bioenergetics mechanism, specifically, changes in mitochondria-related genes linked to specific physiological processes and functions in mitochondria. We acknowledge that experimental variability and missing data are potential threats to reliability. All laboratory work will be handled by a single research assistant and the investigator. Measuring mitochondrial bioenergetics profile will be performed on the same day that lymphocytes are harvested, using the same batch. |
| Further, we use a new approach to measure mitochondrial OXPHOS in fresh isolated human lymphocytes. Specifically, Dr. Hoppel and his research team have developed a method to measure complex III and integrated mitochondrial function from human fibroblasts [63]. The standard laboratory procedure has been optimized and tailored to use of human lymphocytes in order to test the proposed hypothesis. In addition, obtaining data from a control group (age-, race-, and clinical stage-matched prostate cancer patients without XRT) will enable us to control for the influence of confounding variables on outcome measures. |
Conclusion
|
| Achieving the aims of this hypothesis-testing project will establish the molecular-genetic mechanism of radiation-induced fatigue in prostate cancer patients receiving XRT. The downregulation of BCS1L, a defect in complex III, and ATP depletion and ROS production in lymphocytes will need to be verified with a large sample in order to identify early biomarkers prognostic for radiation-induced fatigue in clinical settings. Nonetheless, our results will provide the foundation for interventions targeting cancer-related fatigue. |
| This research project is an essential step in pursuing a novel hypothesis designed to reveal the physiologic mechanisms of cancer-related fatigue, a ubiquitous and significant cause of patient distress. The results have the potential for identifying targets for pharmacological and/or nutraceutical interventions and initiating a new direction for design of interventions for cancer-related fatigue. |
Funding
|
| This research project is funded by Oncology Nursing Society Foundation-Nursing Research Grant RE01. |
Conflicts of interest
|
| The authors report no conflicts of interest. |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |
| |









