Patrícia Maurer1, Vanusa Manfredini2, Rafael Noal Moresco3, Matias Nunes Frizzo4, Ivana Beatrice Mânica da Cruz3, Jacqueline da Costa Escobar Piccoli1,2*
Universidade Federal Do Pampa, Programa de Pós Graduação em Ciências Farmacêuticas, Uruguaiana, RS, Brazil
Universidade Federal Do Pampa, Programa de Pós Graduação em Bioquímica, Uruguaiana, RS, Brazil
Universidade Federal de Santa Maria, Programa de Pós Graduação em Farmacologia, Santa Maria, RS, Brazil
Instituto Cenecista de Ensino Superior de Santo Ângelo, Curso de Biomedicina, Santo Ângelo, RS, Brazil
Corresponding Author:
Jacqueline Piccoli
Universidade Federal do Pampa, BR 472, Km 592
Postal Code 97500970, Uruguaiana, Brazil
Tel: 55 55 39110200
E-mail: jacquelinepiccoli@unipampa.edu.br
Received date: November 02, 2015; Accepted date: November 22, 2015; Published date: December 07, 2015
Citation: Piccoli J, Association between Nitrite / Nitrate and Metabolic Risk in Blacks. Arch Med. 2015, 8:1.
Keywords
Nitric oxide, Negroid race, Oxidative stress, Cardiovascular, Afrodescendants
Introduction
Outside of Africa, Brazil is the country that has the largest population of blacks. This population is known to be more socially and economically vulnerable than other Brazilians, with a lower life expectancy and susceptibility to diseases and health problems [1]. Blacks have a higher cardiovascular risk, even at a younger age, [2] however, paradoxically, they have lower rates of metabolic syndrome (MetS) [3]. The reason for this can diminished the influence of some components of the metabolic syndrome in risk prediction [4].
Nitric oxide is a free radical that is implicated in a wide variety of physiological and pathological conditions [5]. It has an important role as vasodilatador, vasoprotective, bronchodilatador and inflammatory mediator [6-8].
Its metabolites, nitrite/nitrate (NOx), have emerged for the purpose of monitoring the health status of patients with cardiovascular diseases and appears to be promisingly used in the clinical field for the assessment of the pathological state [9]. However, there are no studies that evaluate the NOx levels in the black population, which is a population at high cardiometabolic risk and with peculiar health characteristics. Thus, this study aims to analyze the levels of nitric oxide metabolites and its applicability as a cardiometabolic biomarker in a population of Brazilian blacks.
Materials and Methods
The study was approved by the Ethics Research Committee/ National Research Ethics Commission protocol number 977.827, according to the Helsinki Declaration.
Recruitment of participants
We recruited and enrolled 202 self-declaring blacks (i.e. blacks and browns) as volunteers in southern Brazil (Uruguaiana/ Rio Grande do Sul). All of the subjects signed an informed consent. The only ones to be excluded were those who present hemoglobinopathies and who are under 18 years of age.
Anthropometric measurements
The participants completed an examination with standardized measurements of weight, height, waist circumference (WC) (measured in average distance between the last rib and iliac crest around the navel) and hip circumference (HC) (measured at the maximum extension of the buttocks). From these measurements we calculated each participant’s BMI, waist-to-height ratio (WHtR) and waist-to-hip ratio (WHR). Body mass index (BMI) was calculated by dividing the weight (kg) with height (m2) and the classification was based on standard clinical definitions: normal weight 18.5-24.9 kg/m2; overweight 25.0-29.9 kg/m2 and obese ≥ 30 kg/m2. The guidelines of the International Diabetes Federation (IDF) were used for the diagnosis of Metabolic Syndrome (MetS) [10].
Sample preparation and measures
Venous blood was collected from all subjects after an overnight fast into tubes with EDTA or no anticoagulants. The samples were immediately centrifuged at 1500 rpm for 10 minutes at 4°C. The serum was utilized for biochemical analysis and Total Antioxidant Capacity (TAC), Total Oxidant Status (TOS), Ischemia Modified Albumin (IMA) and NOx. Erythrocytes were used for activity superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). Plasma EDTA was used for the measurement of lipid peroxidation and protein carbonylation.
Fasting glucose, total cholesterol, HDL cholesterol, triglycerides, uric acid, urea and creatinine were performed by use of standard methods on ChemWell T (Labtest Diagnóstica SA, Minas Gerais, Brazil). LDL cholesterol was estimated by Friedwald’s formula [11]. NOx levels were determined indirectly at Cobas Mira (Roche Diagnostics, Basel, Switzerland) by quantification of their metabolites (nitrates and nitrites), based on the Griess reaction, by a method previously described and validated by Tatsch et al. [12].
The glomerular filtration rate (GFR) was estimated by the Cockcroft-Gault equation [13]. TAC and TOS levels were calculated by using a new automated measurement technique described by Erel [14,15]. Malondialdehyde (MDA) levels for the measurement of lipid peroxidation were determined by the previously described spectrometric method [16]. Protein carbonyls were measured by Levine’s method [17]. Catalase activity was determined in accordance with the method that was already described [18]. RANSOD and RANSEL kits (RANDOX Laboratories Ltd., UK) were used to determine the activity of SOD and GPX enzymes following the manufacturer’s protocol.
Statistical analysis
Data was statistically analyzed by using SPSS 20.0 software and the results are present as a mean ± standard deviation (SD). The NOx cutoff points were determined by the 50th percentile of the sample. The Student’s t-test was used to determine the differences between the mean values of these parameters and the NOx cutoff point. For bivariate analysis between categorical variables and the cutoff point, we used the chi-squared test. Multivariate analysis with logistic regression (Backward Wald method) was used to test interfering factors. The statistical significance was assumed at p<0.05.
Results
Two hundred two people participated in the research between March and October of 2014. Most of the volunteers were (162, 80.2%) between 18 to 90 years of age. These individuals reported their race/color as black (57.4%) or brown (‘‘pardo”, in Brazil) (42.6%). Demographic, anthropometric and blood pressure characteristics are shown in Table 1. Considering the influence of social and environmental factors in hypertension, presented in Table 2 some lifestyle indicators.
| Characteristics |
Mean |
SD |
| Age (years) |
46.4 |
14.7 |
| Peso (kg) |
78.1 |
15.2 |
| BMI (kg/m2) |
30.1 |
5.8 |
| WC (cm) |
98.9 |
12.9 |
| HC (cm) |
108.6 |
11.5 |
| WHR |
0.91 |
0.84 |
| WHtR |
0.61 |
0.08 |
| SBP (mmHg) |
133.5 |
24.6 |
| DBP (mmHg) |
86.2 |
17.3 |
BMI: Body Mass Index, WC: Waist Circumference, HC: Hip Circumference WHR: Waist-Hip Ratio, WHtR: Waist-to-Height Ratio, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, SD: Standard Deviation.
Figure 1: Presence of components of metabolic syndrome in percentiles NOX.
* indicates significant difference (p=0.016), MetS= Metabolic Syndrome.
| |
N |
% |
| Marital Status |
| Married |
95 |
47.0 |
| Single |
65 |
32.1 |
| Widower |
22 |
10.9 |
| Divorced |
20 |
10.0 |
| Functional Situation |
| Worker |
103 |
51.0 |
| Retired |
40 |
19.8 |
| Student |
9 |
4.5 |
| Unemployed/Housewife |
50 |
24.7 |
| Education |
| Illiterate |
12 |
5.9 |
| Elementary school |
90 |
44.5 |
| Middle school |
20 |
9.9 |
| Incomplete High school |
21 |
10.4 |
| Complete High school |
43 |
21.3 |
| College |
9 |
4.5 |
| Postgraduate |
7 |
3.5 |
Table 2: Lifestyle indicators and functional features.
The participants were divided according to their NOx value in percentiles to identify the relationship according to their different metabolic markers. The dosage of the NOx ranged between 28.1 and 598.0 μmol/L and the median, which corresponds to the 50th percentile (50p), was 122.3 μmol/L, thus the sample was divided into 101 (50%) subjects with NOx ≥ 50p and 101 (50%) with NOx <50p. To evaluate the influence of NOx and diseases, Table 3 presents the baseline diseases of the black population.
| Disease |
N (%) |
NOx ≥ 50p
N (%) |
NOx <50p
N (%) |
Odds Ratio (IC 95%) |
| Hypertension |
108 (53.5) |
57 (52.8) |
51 (47.2) |
1.2 (0.7-2.2) |
| Diabetes |
32 (15.8) |
15 (46.9) |
17 (53.1) |
0.8 (0.4-1.8) |
| Dyslipidemia |
45 (22.2) |
29 (64.4)a |
16 (35.6) |
2.0 (1.0-4.0) |
| Acute Myocardial Infarction |
6 (3.0) |
2 (33.3) |
4 (66.7) |
0.4 (0.1-2.7) |
| Angina |
50 (24.7) |
25 (50.0) |
25 (50.0) |
1.0 (0.5-1.9) |
| Stroke |
8 (3.9) |
6 (75.0) |
2 (25.0) |
3.1 (0.6-15.9) |
| Smoke |
38 (18.8) |
20 (52.6) |
18 (47.4) |
1.1 (0.6-2.3) |
| Use of alcohol |
52 (25.7) |
27 (50.0) |
27 (50.0) |
1.0 (0.5-1.9) |
| Sedentary |
131 (64.8) |
62 (47.3) |
69 (52.7) |
0.7 (0.4-1.3) |
a= p<0.05
Table 3: Baseline diseases and differences by percentile value of 50% NOx.
The prevalence of MetS by the IDF criteria was 61% in this population. In the analysis of each MetS criteria individually and their relationship to the percentile 50% of NOx using the chi-square test (Figure 1), only the obesity criterion (given by measuring the WC) was statistically significant with the lower percentile values 50% of NOx (RR=1.71, CI 1.03-2.85, p=0.016).
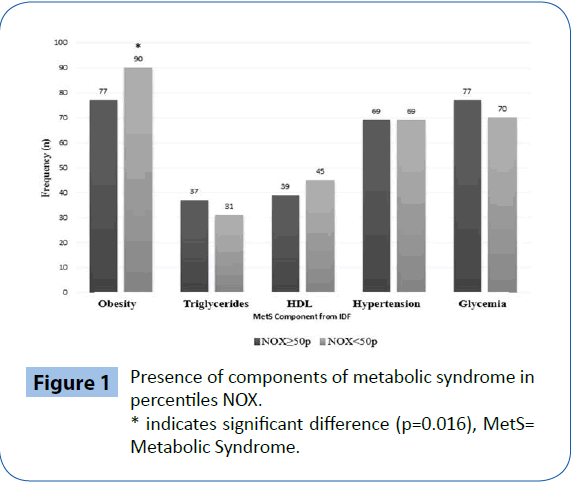
Figure 1: Presence of components of metabolic syndrome in percentiles NOX. * indicates significant difference (p=0.016), MetS= Metabolic Syndrome.
The NOx percentiles were crossed with the anthropometric markers, and Table 4 shows that blacks with lower levels of NOx (below the 50th percentile) had significantly higher BMI, WC and HC. The biochemical, inflammatory and oxidative parameters were analyzed and compared with the percentile of nitric oxide metabolites and the results are shown in Table 5. The NOx ≥ 50p group had higher levels of glucose (p=0.049), triglycerides (p=0.046), albumin (p=0.034), Uric acid (p=0.019) and urea (p=0.052) than the NOx<50p group. The analysis of logistic regression showed that the variables waist circumference and carbonyl levels were related with the NOx ≥ 50p group independently of sex, age and all other variables tested.
| |
Nox ≥ 50p
(mean ± sd) |
NOx < 50p
(mean ± sd) |
p |
| BMI (kg/m²) |
29.08 ± 5.72 |
31.07 ± 5.75 |
0.01 |
| WC (cm) |
97.03 ± 12.89 |
100.86 ± 12.79 |
0.03 |
| HC (cm) |
106.89 ± 11.22 |
110.22 ± 11.59 |
0.04 |
| WHR |
0.91 ± 0.09 |
0.91 ± 0.07 |
0.68 |
| SBP (mmHg) |
132.60 ± 26.29 |
134.45 ±2 3.01 |
0.59 |
| DBP (mmHg) |
85.95 ± 18.28 |
86.50 ± 16.42 |
0.82 |
BMI: Body Mass Index, WC: Waist Circumference, HC: Hip Circumference, WHR: Waist-Hip Ratio, WHtR: Waist-to-Height Ratio, SBP: Systolic Blood Pressure, DBP:Diastolic Blood Pressure, SD: Standard Deviation, Ns: Not significant
Table 4: Obesity and blood pressure criteria compared with percentiles of NOx.
| Parameter |
NOx ≥ 50p
(mean ± sd) |
NOx < 50p
(mean ± sd) |
P |
| Glucose (mg/dL) |
131.35 ± 50.56 |
118.84 ± 38.42 |
0.04 |
| Total Cholesterol (mg/dL) |
184.81 ± 40.17 |
185.50 ± 44.91 |
0.90 |
| HDL Cholesterol (mg/dL) |
50.91 ± 13.30 |
49.17 ± 12.36 |
0.33 |
| LDL Cholesterol (mg/dL) |
104.36 ± 14.78 |
112.55 ± 39.71 |
0.12 |
| Triglycerides (mg/dL) |
150.32 ± 99.67 |
126.65 ± 63.58 |
0.04 |
| Albumin (g/dL) |
4.08 ± 0.66 |
3.89 ± 0.56 |
0.03 |
| GFR (mL/min/1,73m²) |
121.43 ± 89.75 |
122.25 ± 44.44 |
0.93 |
| Uric acid (mg/dL) |
5.71 ± 2.01 |
5.12 ± 1.27 |
0.01 |
| Urea (mg/dL) |
26.06 ± 15.17 |
22.67±6.85 |
0.05 |
| Creatinin (mg/dL) |
0.84±0.30 |
0.79 ± 0.16 |
0.24 |
| AST (U/L) |
27.95 ± 8.18 |
29.41 ± 12.43 |
0.35 |
| ALT (U/L) |
11.28 ± 4.84 |
12.46 ± 7.39 |
0.20 |
| GGT (U/L) |
28.34 ± 38.41 |
34.09 ± 34.60 |
0.29 |
| CRP (mg/L) |
9.07 ± 6.21 |
9.28 ± 7.17 |
0.81 |
| TAC (mmol Trolox Equivalent/L) |
0.28 ± 0.14 |
0.29 ± 0.15 |
0.68 |
| TOS (mmol Trolox Equivalent/L) |
83.96 ± 49.24 |
85.19 ± 50.33 |
0.86 |
| MDA (µmol MDA/ mL) |
28.80 ± 11.30 |
27.56 ± 12.96 |
0.50 |
| Carbonyl (nmol/g prot) |
5.00 ± 2.12 |
6.33 ± 2.48 |
<0.01 |
| CAT (U/mg Hb) |
57.52 ± 14.37 |
54.79 ± 13.72 |
0.17 |
| SOD (U/mg prot) |
0.27 ± 0.98 |
0.19 ± 0.04 |
0.39 |
| GPx (U/mg prot) |
1725.30 ± 210.08 |
1746.25 ± 139.78 |
0.40 |
GFR: Glomerular Filtration Rate, AST: Aspartate Aminotransferase, ALT: Alanine aminotransferase, GGT: Gamma-Glutamyl Transferase, CRP: C-reactive Protein, TAC: Total Antioxidante Capacity, TOS: Total Oxidant Status, MDA:Malondialdehyde, CAT:Catalase, SOD:Superoxide Dismutase, GPx: Glutathione peroxidase
Table 5: Biochemical, inflammatory and oxidative parameters versus values in NOx percentile among blacks.
Discussion
As shown in Table 1, the studied black population consists of adults in whom obesity criteria are outside the normal range. The average body mass index (BMI) was 30.1kg/m2, which characterizes obesity degree I. The obesity rate for blacks is known to be higher than in whites. One study showed that the prevalence of obesity among blacks and whites was 23.3% and 11.2% respectively, while the overweight category included 76.2% of blacks and 67.2% of whites [19].
The mean values of waist circumference (98.9 cm) are above the limits recommended by the IDF abdominal adiposity, indicating a large deposit of abdominal fat. Increased waist circumference values have been associated with increasing age, particularly in a study of predominantly Brazilian black women [20]. When stratifying the waist circumference measures by sex, the average WC for men was 95.8 ± 12.4 cm and 99.8 ± 13.0 cm (p=0.08) for women which, was not significant, then it is a homogeneous population. Besides, the model of logistic regression showed that waist circumference was variable independently associated with NOx ≥ 50p group, indicating that elevated levels of nitric oxide has a close relationship with obesity indicators.
The average value of WHR=0.91 indicates cardiometabolic risk. The values of WHR=0.85 for men and WHR=0.90 for women as the cutoff point were considered compatible with specificity between high and moderate and useful as predictors of hypertension [21]. WHR by gender was 0.94 ± 0.10 cm for men and 0.90 ± 0.08 cm for women (p=0.08), thus highlighting the increase of this anthropometric relationship in this population, regardless of gender.
The average blood pressure was 133 × 86 mmHg, which was classified as borderline according to the Brazilian Society of Hypertension [22]. A population-based study in our state (Rio Grande do Sul) with nine hundred and eighteen adults showed a 33.7% prevalence of hypertension, and 49.8% of such a population was unaware of it [23]. This is an important finding, since hypertension is a non-communicable chronic disease with high morbidity and mortality, usually asymptomatic, and the lack of knowledge about it is a challenge, both in Brazil and in the world, for the control of blood pressure [23] [24].
The general data so far shows a population in cardiovascular risk, as seen in the average levels of blood pressure and anthropometrically checked. A study shows that 79% of hypertensive men and 65% of hypertensive women have cardiovascular events as a direct result of overweight, indicating a linear relationship between BMI and blood pressure [25]. It is estimated that a 1% reduction in the BMI would also result in a significant reduction in diabetes, cardiovascular diseases and cancer in the population [26]. Cardiovascular diseases account for a large number of hospitalizations and health care costs in Brazil [26]. Some explanations for the higher prevalence of hypertension among blacks include greater sensitivity to alcohol, increased salt sensitivity and the high renal retention of sodium, as well as differences in phenotypes and genotypes [27].
The marital status of individuals influences self-care and family dynamics [28]. In the health profile, studies related to self-care, healthier lifestyles and better quality of life in married subjects nearly signify the majority of our population (46.8%). More than half of the population (50.2%) are illiterate or have not finished elementary school, thus indicating a low level of education. The low educational level of most of the sample may be related to the results of the functional situation presented by the participants with the monthly income. The average income was 1.5 times the minimum wages (R$788.00; approximately $255.00, ranging from 1 to 10 minimum salaries. Lower income and education are commonly associated with poor access to health, bad quality of care, higher mortality, and, then, higher rates of disease. We observed a high prevalence of hypertension (53.5%) in this sample of the black population, and 31.6% it already had a cardiovascular event. Previous Brazilian studies have found a hypertensive prevalence of 19.8% in Rio Grande do Sul state [29], and prevalence of hypertensive prevalence of 29.9% associated with obesity and black women in Bahia state [30]. In the United States, a study found the prevalence of hypertension among blacks of 40% and whites 22%, indicating great disproportionality [3]. Although we attempted to verify that the NOx value was related to the presence of previous diseases by bivariate chisquare analysis, only dyslipidemia were significantly associated with NOx ≥ 50p (p=0.041). Blacks generally have lower rates of dyslipidemia in comparison to whites, but, in compensation, have higher rates of obesity, hypertension and diabetes [31].
The low bioavailability of NOx can be associated with the reaction of nitric oxide and oxygen with the formation of peroxynitrite, commonly because of inflammatory characteristics and oxidative stress in obesity [32]. Considering the physiological role of NO in the decrease of circulating NOx has detrimental effects, which result in cellular dysfunction and can cause insulin resistance, hypertension, pancreatic β cell dysfunction and diabetes [33].
As shown in Table 5, for glucose values in both percentiles, there is an increased value over that which would be considered suitable for fasting glucose until 100 mg/dL according to the criteria IDF, [10] and only 15.8% of the population had a diagnosis of diabetes. We suggest that these results strongly indicate the possibility of insulin resistance (IR) or prediabetes, whereas a failure in β pancreatic cells or insulin sensitivity may be causing the hyperglycemia that was presented by studied subjects.
An increase in NOx levels and hyperglycemia in patients with diabetes mellitus type II has been observed. The association between diabetes and oxidative stress (i.e., the formation of peroxynitrite) may explain why, even with high levels of NO, vascular resistance and, consequently, endothelial dysfunction can increase [34].
Although the triglyceride values differ, both percentiles are below or near normal value are less than 150 mg/dL. Triglyceride levels in blacks are usually lower, [35] even when other risk factors such as diabetes mellitus type II, cardiovascular disease and IR are present. Namely the absence of increased triglycerides does not imply the absence of cardiovascular risk in this population.
The albumin level decreased and had become associated with the NOx lower than the 50th percentile, although it was still slightly above the normal value (3.5 mg/dL). The hypoalbuminemia may result from liver or kidney disease, could have been associated with increased oxidative stress [36], or is likely to be a link between hyperglycemia and the renal profile presented by the participants. Obese individuals usually have decreased renal blood flow [37], which corroborates the results of urea and uric acid decreased in participants with lower NOx than the 50th percentile, who have shown an association with worse anthropometric indicators; however, the GFR presented for both NOx values indicates the preservation of renal function.
The damage to proteins evidenced by carbonyl was significantly higher in individuals with lower NOx (p<0.01; indirectly proportional). Proteins are immediate targets of oxidative stress and, when oxidation occurs in their side chains, carbonyl groups are produced. These carbonyl groups can be used as protein damage biomarkers, and its accumulation has been observed in several pathologies [38]. The affinity of the superoxide anion with nitric oxide is greater than with superoxide dismutase. This reaction and the formation of peroxynitrite contributed to decreased bioavailability of NO, hence, a lower NOx value, which explains our findings in carbonylation.
This study has some limitations. Diet can affect the levels of circulating nitric oxide metabolites, and the subjects enrolled in our study were not on a specific diet before blood collection. This context is important since ingestion of some functional foods and nutraceuticals have antioxidant effect and may also have beneficial effects on the cardiovascular risk [39,40]. Even, there was a great variety of individuals and results due to the absence of rigorous exclusion criteria (e.g., the presence of previous diseases).
Conclusions
The relationships that are shown between the percentiles of nitrite/nitrate and biochemical parameters, such as inflammatory and oxidative stress, suggest that NOx is a good predictor of cardiometabolic risk, that is strongly associated with biochemical markers such as glucose and triglycerides, as well as measures to obesity that, in itself, represents an increased risk for other diseases.
Furthermore, the NOx dosage is easily adapted to laboratory testing and can be routinely programmed for automatic analyzers, thus requiring very little sample preparation and allowing it to be used in research as well as diagnostic laboratories. It is, also useful in predicting metabolic risk and guiding good treatment measures.
Funding
This research was support by the Brazilian research agency (CNPq: Process 466617/20143). Patricia Maurer receives a fellowship from FAPERGS/Brazil.
7933
References
- NetoJAC, Fonseca GM, Brum IV, Santos JLCT, Rodrigues TCGF, et al. (2015) The National Comprehensive Health Policy for the Black Population: implementation, awareness and socioeconomic aspects from the perspective of this ethnic group. CiencSaudecoletiva 20: 1909-1916.
- Lillie-Blanton M, Maddox TM, Rushing O, Mensah GA (2004) Disparities in cardiac care: rising to the challenge of Healthy People 2010. J Am CollCardiol 44: 503-508.
- Osei K(2010) Metabolic syndrome in blacks: are the criteria right? CurrDiab Rep 10: 199-208.
- Gaillard T, Schuster D, Osei K (2009) Metabolic syndrome in Black people of the African diaspora: the paradox of current classification, definition and criteria. Ethn Dis 19: S2-1-7.
- Mian AI,Aranke M, Bryan NS (2013) Nitric oxide and its metabolites in the critical phase of illness: rapid biomarkers in the making. Open Biochem J 7: 24-32.
- Zhang X, Lynch AI, Davis BR, Ford CE, Boerwinkle E, et al. (2012) Pharmacogenetic association of NOS3 variants with cardiovascular disease in patients with hypertension: the GenHAT study. PLoS One 7: e34217.
- Petrella E,Pignatti L,Neri I,FacchinettiF2 (2014) The l-arginine/nitric oxide pathway is impaired in overweight/obese pregnant women. Pregnancy Hypertens 4: 150-155.
- Konukoglu D,Serin O, Turhan MS (2006) Plasma leptin and its relationship with lipid peroxidation and nitric oxide in obese female patients with or without hypertension. Arch Med Res 37: 602-606.
- Ghasemi A,Zahediasl S, Azizi F (2010) Reference values for serum nitric oxide metabolites in an adult population. ClinBiochem 43: 89-94.
- Alberti KG,Zimmet P, Shaw J (2006) Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469-480.
- FriedewaldWT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. ClinChem 18: 499-502.
- Tatsch E,BochiGV, Pereira Rda S, Kober H, Agertt VA, et al. (2011) A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. ClinBiochem 44: 348-350.
- Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: 31-41.
- Erel O(2005) A new automated colorimetric method for measuring total oxidant status. ClinBiochem 38: 1103-1111.
- Erel O(2004) A novel automated method to measure total antioxidant response against potent free radical reactions. ClinBiochem 37: 112-119.
- Ohkawa H, Ohishi H, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Levine RL(2002) Carbonyl modified proteins in cellular regulation, aging, and disease. Free RadicBiol Med 32: 790-796.
- Kumanyika SK, Whitt-Glover MC, Haire-Joshu D (2014) What works for obesity prevention and treatment in black Americans? Research directions. Obes Rev 15 Suppl 4: 204-212.
- Oliveira LC, West LEM, AraujoEA, BritoJS, SobrinhoCLN (2015) Prevalencia de adiposidade abdominal emadultos de São Francisco do Conde, Bahia, Brasil, 2010. EpidemiolServSaude24: 135-144.
- Pereira RA,Sichieri R, MarinsVM (1999) [Waist:hips girth ratio as a predictor of arterial hypertension]. Cad SaudePublica 15: 333-344.
- Cardologia SB de, Hipertensao SB de, Nefrologia SB de (2010) VI DiretrizesBrasileiras de Hipertensao. Arq Bras Cardiol 95: 1-51.
- Gus I,Harzheim E, Zaslavsky C, Medina C, Gus M (2004) Prevalence, awareness, and control of systemic arterial hypertension in the state of Rio Grande do Sul. Arq Bras Cardiol 83: 429-433.
- Nobre F, Coelho EB, Lopes PC, GeleileteTJM (2013) Hipertensao arterial sistemicaprimaria. Medicina 46: 256-272.
- Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA (2011) Impact of obesity on cardiovascular disease. Med Clin North Am 95: 919-937.
- Bahia L, CoutinhoESF, Barufaldi LA, Abreu GA, Malhao TA, et al. (2012) The costs of overweight and obesity-related diseases in the Brazilian public health system: cross-sectional study. BMC Public Health 12: 1-7.
- Fuchs FD (2011) Why do black Americans have higher prevalence of hypertension?: an enigma still unsolved. Hypertension 57: 379-380.
- MiranziSSC, Ferreira FS, IwanotoHH, Pereira GA, Miranzi MAS (2008) Qualidade de vida de indivíduos com diabetes mellitus e hipertensãoacompanhadosporumaequipe de saude da familia. TextoContextoEnferm 17: 672-679.
- Piccini RX,Victora CG (1994) [Systemic arterial hypertension in a urban area of southern Brazil: prevalence and risk factors]. Rev SaudePublica 28: 261-267.
- Lessa I,Magalhães L, AraújoMJ, de Almeida Filho N, Aquino E, et al. (2006) Arterial hypertension in the adult population of Salvador (BA)--Brazil. Arq Bras Cardiol 87: 747-756.
- Sumner AE(2009) Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr 155: S7.
- FensterCP, Darley-UsmarVM, Landar AL, Gower BA, WeinsierRL, et al. (2004) Weight loss and race modulate nitric oxide metabolism in overweight women. Free RadicBiol Med 37: 695-702.
- Krause M, Rodrigues-Krause J, O´Hagan C, De Vito G, Boreham C, et al. (2012) Differential nitric oxide levels in the blood and skeletal muscle of type 2 diabetic subjects may be consequence of adiposity: a preliminary study. Metabolism 61: 1528-1537.
- Yugar-Toledo JC, Tanus-Santos JE, Sabha M, Sousa MG, Cittadino M, et al. (2004) Uncontrolled hypertension, uncompensated type II diabetes, and smoking have different patterns of vascular dysfunction. Chest 125: 823-830.
- Yu SS, Castillo DC, Courville AB, Sumner AE (2012) The triglyceride paradox in people of African descent. MetabSyndrRelatDisord 10: 77-82.
- Gutiérrez OM,Khodneva YA, Muntner P, Rizk DV, McClellan WM, et al. (2013) Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA 310: 706-714.
- Reisin E, Owen J (2015) Treatment: special conditions. Metabolic syndrome: obesity and the hypertension connection. J Am SocHypertens 9: 156-159.
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. ClinChimActa 329: 23-38.
- Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, et al. (2014). Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods 6: 11-32.
- Ciccone MM,Cortese F,Gesualdo M,Carbonara S,Zito A, et al. (2013) Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm 2013: 782137.







