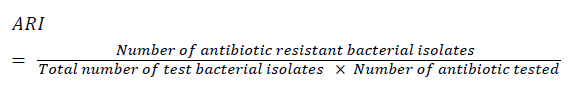Keywords
Eye cosmetics; Bacterial contaminants; Antibiogram; Zone diameter of inhibition; Multiple antibiotic resistance index
Introduction
Microbial contamination of cosmetics is a major public health problem [1,2], and also the cause of concern to the industries, the users as well the clinicians. In the current ages, various cosmetics, including eye-cosmetics (eye liners, eye shadow, mascara, eyelash curlers, kohl) are in use in order to improve self-esteem and appearance, and among those kohl (also called ‘kajal’ in Bengalee) is a popular eye care product, the use of which has been reported since ancient times. In India, the use of kohl in pediatric age is a common practice to keep the eyes cool, clean and with improved vision [3], while the older infants, children and women apply kohl for beautification and to protect and treat eye diseases. But such agents including the eye-cosmetics may have the capability to serve as the vehicles of bacterial infection into the eyes of the users if contaminated products are used, or can disseminate the infection into others when such agents are shared or misused [4]. Campana et al. [5] studied commercially available cosmetics in order to verify the possible microbial contamination during their use by the consumers. Orus and Leranzo [6] reported the isolation of gram-positive bacteria such as Staphylococcus aureus, S. epidermidis as well as gramnegative bacteria: Pseudomonas aeruginosa, Klebsiella pneumonia and Escherichia coli from mascara and eye pencil. Dawson and Reinhardt [7] reported bacterial contamination of eye pencil with the genera Staphylococcus, Micrococcus, Bacillus, Moraxella, Acinetobacter and Pseudomonas. The bacterial strains isolated from different cosmetics including ‘kajal’ were identified as E. coli, Staphylococcus sp. and Bacillus sp. and the isolates were found resistant to one or more antibiotic tested such as chloramphenicol (CM), tetracycline (TC) and streptomycin (SM) [8].
Abdelaziz et al. [9] reported a large number of potential pathogenic bacteria such as P. aeruginosa, Citrobacter freundii, K. pneumonia, E. coli, Enterobacter agglomerans, S. epidermidis and Micrococcus sp. from different cosmetics including eye shadow and mascara. Bacteria such as Bacillus spp., Staphylococcus spp., Pseudomonas spp., P. vulgaris and Serratia marcescens have been recovered from unused and inuse samples of Al-Kohl [10]. The neonates on application of kohl got infection with microorganisms in their conjunctivae [11]. Akrayi [12] isolated gram-positive (S. aureus, S. epidermidis and S. capitis) and gram-negative (E. coli) bacteria from the eye lids of eye-cosmetic users and natural eye liner users. Baqer et al. [13] isolated various bacterial strains such as Proteus, E. coli, Shigella, Citrobacter, Klebseilla, P. aeruginosa, S. aureus and S. epidermidis from used cosmetic samples including mascara. Such bacterial contamination of the cosmetics may cause spoilage of the products [14], or lead to human illness from simple skin infection, conjunctivitis and allergy to keratitis, whole body inflammation and systemic blood infection [5]. However, scientific studies on bacterial contamination of eye-cosmetics and the antibiotic susceptibility of the isolated bacteria are lacking in our part of the globe. Therefore, the current study has been undertaken to isolate and identify the potential bacterial strains from different types of commercially available eye-cosmetics in Malda (West Bengal state, India), and to determine the antibiotic resistance patterns of the bacteria involved.
Methods
Sampling sites and sample collection
A total of 10 randomly selected eye cosmetic (EC) samples: koh-1, kohl-2 and kohl-3; mascara-1 and mascara-2; eye shadow-1 and eye shadow-2; eye liner-1, eye liner-2 and eye liner-3 were collected from Malda town of the West Bengal state, India, and were subjected for bacteriological processing.
Isolation and identification of bacteria
Each of the ECs procured was inoculated into nutrient broth (Hi-Media, India), and following incubation at 37°C for 24 h, a loop-full of the broth cultures (from each sample) were streaked on the surface of blood agar, MacConkey agar, cetrimide agar and nutrient agar (Hi-Media, India), and incubated for 24 h at 37°C. Single and discrete (morphologically different) colonies grown on various agar plates were stored in cystine tryptone agar (Hi-Media, India) stabs. The bacterial strains isolated were identified following gram-staining, biochemical tests (catalase, oxidase, urease, nitrate reduction, gelatine hydrolysis and IMViC) and sugar fermentation [15,16].
Antibiotic susceptibility
The antibiotic susceptibility for the bacterial strains from ECs was determined by disc diffusion method [17], using Mueller- Hinton agar (Hi-Media, India) plates, which were swabinoculated with overnight grown broth culture of the isolates, and were incubated with ten antibiotic discs (Hi-Media, India): ciprofloxacin (CIP), vancomycin (VA), nalidixic acid (NA), meropenem (MRP), ampicillin (AMP), cefpodoxime (CPD), cefotaxime (CTX), trimethoprim (TR), gentamycin (GEN) and amikacin (AK). The results, in terms of zone diameter of inhibition (ZDI) obtained around each of the antibiotic discs for the isolates, were interpreted following the criteria of the Clinical Laboratory Standards Institute [18], and the isolates were categorized as resistant, sensitive or intermediately susceptible.
Determination of antibiotic resistance and multiple antibiotic resistance indices
The antibiotic resistance index (ARI), and multiple antibiotic resistance (MAR) index for all the isolated bacteria were calculated as follows [19-21]:
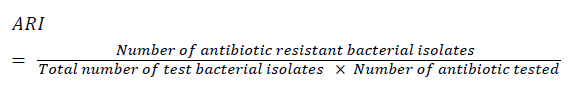
and

and interpreted according to Krumperman [19]: MAR index ≤ 0.2 was considered low risk, and ≥ 0.2 was indicated as high risk.
Results
Among 10 ECs collected, 8 showed bacterial contamination as per the microbial culture in various media; two (eye liner-1 and eye liner-2) were free from bacterial contamination. Among the isolated bacteria (n=9), 6 were gram-positive [strain code: C3(1)K, C5(A), C5(B), C7(1), C9(A) and C4(A)C, recovered respectively from kohl-1, eye shadaw-1, eye shadow-2, kohl-3, eye liner-3 and mascara-2]. The remaining 3 were gram-negative [strain code: C6(B)D, C8(A) and C2(A)A, which were isolated from kohl-2, liner-3 and mascara-1], respectively. All the bacterial isolates obtained were rod shaped.
In TSI, 6 strains [C5(A), C5(B), C7(1), C4(A)C, C6(B)D and C8(A)] showed acid butt (yellow) and alkali slant (pink), while the C2(A)A strain had red butt and red slant, and the two gram-positive small rod shaped bacteria with strain code C3(1)K and C9(A) had acid butt (yellow) and acid slant (yellow); no strain was found positive for gas (CO2) and H2S production. Among the gram-negative bacilli, the C2(A)A strain did not ferment any sugars used in the study, while the other 2 strains [C6(B)D and C8(A)] fermented glucose, but did not ferment lactose, mannitol, sorbitol and xylose. Among the grampositive bacilli, 3 strains: C5(A), C5(B) and C7(1) fermented sucrose, sorbitol, xylose, glucose but did not ferment mannitol and lactose, while 2 strains [C3(1)K and C9(A)] fermented glucose, sucrose, rhamnose, sorbitol, mannitol and lactose, but did not ferment xylose. The biochemical test results for the isolated eye-cosmetic bacteria are shown in Table 1.
| Strain |
CAT |
OXI |
IND |
CIT |
URE |
MR |
VP |
NIT |
GEL |
| C3(1)K |
+ |
- |
- |
- |
+ |
+ |
+ |
- |
+ |
| C2(A)A |
+ |
+ |
- |
+ |
- |
- |
- |
- |
- |
| C4(A)C |
+ |
+ |
- |
+ |
+ |
- |
+ |
+ |
+ |
| C5(A) |
+ |
+ |
- |
- |
- |
- |
+ |
+ |
+ |
| C5(B) |
+ |
+ |
- |
- |
- |
- |
+ |
+ |
+ |
| C6(B)D |
+ |
+ |
- |
+ |
+ |
+ |
- |
- |
+ |
| C7(1) |
+ |
+ |
- |
- |
- |
- |
+ |
+ |
+ |
| C8(A) |
+ |
+ |
- |
+ |
+ |
+ |
- |
- |
+ |
| C9(A) |
+ |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
Table 1 Biochemical features of the isolated eye-cosmetic bacteria. CAT: Catalase; CIT: Citrate; GEL: Gelatinase; IND: Indole; MR:Methyl red; NIT: Nitrate; OXI: Oxidase; URE: Urease; VP: Voges-Proskaur
Based upon the cultural characteristics (colony morphology, haemolytic activity and pigment production), gram-staining (cell shape), biochemical including TSI test results and sugar fermentation patterns of the eye-cosmetic bacteria, their identities are represented in Table 2.
| Strain code |
Strain identity |
| C3(1)K |
Listeriamonocytogenes |
| C2(A)A |
Pseudomonus aeruginosa |
| C4(A)C |
Bacillussp. |
| C5(A) |
Bacilluscereus |
| C5(B) |
Bacilluscereus |
| C6(B)D |
Chromobacterium violaecium |
| C7(1) |
Bacilluscereus |
| C8(A) |
Chromobacterium violaecium |
| C9(A) |
Listeriamonocytogenes |
Table 2: Identity of the bacterial isolates from different eyecosmetics
The antibiotic susceptibility patterns of the isolated bacteria are represented in Figure 1.

Figure 1: Antibiotic resistance patterns of eye-cosmetic bacteria: (A) P. aeruginosa, (B) L. monocytogenes, (C) C. violaecium, (D) Bacillus spp. AK: amikacin; AMP: ampicillin; CIP: ciprofloxacin; CPD: cefpodoxime; CTX: cefotaxime; GEN: gentamycin; MRP:meropenem; NA: nalidixic acid; TR: trimethoprim; VA: vancomycin; ZDI: zone diameter of inhibition.
The Bacillus sp. was sensitive to all the test antibiotics having ZDIs 19-40 mm; the three strains of B. cereus, such as (C5(A), C5(B) and C7(1), had ZDIs 21-55 mm, 21-54 mm and 20-50 mm, respectively. The isolated P. aeruginosa showed resistance to five antibiotics, with 6 mm ZDIs against VA, AMP, CPD and TR, while 10 mm against NA, and thus highest resistance was displayed by P. aeruginosa (Figure 2).

Figure 2: Pseudomonas aeruginosa on nutrient agar plates. The isolate produced characteristic (greenish yellow colour) pigment, and showed resistance to five antibiotics. AMP: ampicillin; CPD: cefpodoxime; NA: nalidixic acid; TR: trimethoprim; VA: vancomycin.
The C. violaecium C6(B)D and C8(A) strains had resistance to AMP, CPD and TR (ZDIs 6 mm, for each) and to VA (ZDI 13 mm), and the L. monocytogenes C3(1)K and C9(A) strains had resistance to CPD, VA and NA (ZDIs 6-12 mm). The MAR indices ranged 0.3-0.5, among the antibiotic resistant bacteria (Figure 3); the overall antibiotic resistance index was 0.055, for the isolated bacteria.

Figure 3: Multiple antibiotic resistance (MAR) indices of various eye-cosmetic bacterial isolates. C3(1)K and C9(A): Listeria monocytogenes; C6(B)D and C8(A): Chromobacterium violaecium; C2(A)A: Pseudomonus aeruginosa. The digits represented within the figure denote the MAR indices (0.3-0.5).
Discussion
Contamination with pathogenic bacteria of food as well as pharmaceuticals is known [20], and the isolation of potential bacterial pathogens from cosmetics, including eye-cosmetics is not uncommon [2]. Different authors from different parts of the globe isolated bacteria from various types of eyecosmetics, and identified the contaminants as S. aureus, S. epidermidis, P. aeruginosa, Citrobacter freundii, K. pneumonia, E. coli, Micrococcus sp., Bacillus spp., Shigella and Citrobacter, by phenotypic characterization [6,8,12]. As has been reported by Abdelaziz et al. [9], the eye shadow and mascara samples were heavily contaminated with gram-positive cocci as well as gram-negative bacteria including P. aeruginosa, C. freundii, K. pneumonia, E. coli, Enterobacter agglomerans, S. epidermidis and Micrococcus sp. Baqer et al. [13] reported about the isolation of bacteria, such as Proteus, E. coli, Shigella, Citrobacter, Klebseilla, P. aeruginosa, S. aureus and S. epidermidis from mascara, face sponge and the using brushers. The commonest gram-negative ocular bacterial pathogen included Ps. aeruginosa contaminating ophthalmic solutions (eye drops), eye cosmetics and any other substances having a bit of organic carbon [21], as the source of food and energy. In the current study, the bacterial contaminants of different eyecosmetics included P. aeruginosa, C. violaceum, L. monocytogenes and Bacillus sp., including B. cereus. The isolated bacteria were identified by phenotypic characterization (gram staining, colony morphology, biochemical features and sugar fermentation capacity). The C. violaceum isolates in the present study produced light pigment on blood agar plate, and the strains were oxidase positive, however, tested negative for arginine dihydrolase. This current finding was in accordance with the results of Lima-Bittencourt et al. [22], who reported arginine dihydrolase negative C. violaceum isolates along with the arginine dihydrolase positive strains. The C. violaceum generally produce pink/violet pigment on agar plates; however, it has been reported that pigmentation is not a vital feature in the characterization of the genus C. violaceum [23]. It has also been recorded in some cases that non-pigmented variants develop following subcultures of the pigmented isolates [24]. The eyes might be exposed to various types of eye-cosmetics including liner, shadow, blusher, foundation, as well as kohl and mascara with bacterial contamination. This is due to the fact that the preservatives contained in such cosmetics are insufficient, or are of poor quality so as to prevent colonization of potential bacterial pathogens, which in turn cause damage to the eyes, and spoilage of the cosmetic products too.
In the present communication, except Ps. aeruginosa, the all isolated bacteria had β-haemolysis capacity in vitro, and thus potentially cause pathogenesis on infection through the use of eye-cosmetics in the community (sharing the cosmetics having bacterial contamination). From 58 test positive samples, 32 samples of eye drops were found to be contaminated with Bacillus spp. (55.1%), and out of 57 Bacillus isolates, 41 (71.9%) produced different levels of haemolysins [20]. Das et al. [14] isolated Bacillus sp., and considered that the bacterium may be responsible for spoilage and unpleasant smell of cosmetic products. Use of cosmetics with microbial contamination has been associated with various diseases: Clostridium tetani infections attributed to the use of a talcum powder [25], and clinical eye infection due to the transmission and persistence of microorganisms in eye cosmetics [26] have been demonstrated. Numerous cases of eye infections and the loss of vision were reported to be caused by the contaminated cosmetic products with P. aeruginosa [27]. The investigation for microbial contamination of cosmetics has also been reported [28], and death due to the use of such materials contaminated with bacteria has been recorded.
The C. violaceum is found in soil and water; reports suggest the bacterium as an emerging pathogen having the capacity to cause fatal infection (skin and localized infection to septicaemia and lesion) in humans in the tropics and subtropics [29-31]. In the current study, its (C. violaceum) presence in the eye cosmetic might be a potential source of human eye infection. Among the genus Listeria, only L. monocytogenes is consistently associated with human illness, called listeriosis. B. cereus is ubiquitous in nature, the spores of which are heat-, desiccation-, alcohol- and low-pH (1.5) resistant [32], and hence can be isolated from soil, water, air and dust, and occurs in a range of products used by the customers including foodstuffs. The B. cereus strains are associated with human illness like food poisoning (emetic syndrome and the diarrhoeal syndrome) [33,34] as well as some more severe infection including endophthalmitis [35]. Ps. aeruginosa is widely distributed in the environment as well as in living hosts, and opportunistically cause severe corneal infection [36,37], and is regarded as the most common pathogen causing bacterial keratitis that progress rapidly and thus results in permanent loss of vision [38-40]. The bacterium P. aeruginosais had been used, among others, as an indicator of the official assessment of the effectiveness of cosmetics preservation [41]. P. aeruginosa is the etiologic agent of infections to humans, such as that of the cornea and conjunctiva leading to various forms of inflammation, and serious infections of the eye-ball. Beside this, the pathogen causes UTI and lung infections, infection to burn wound, surgical sites, heart muscles and central nervous system infection [41]. In the current study, the all isolated bacteriabeside causing eye and skin infection-can potentially cause bacteraemia, septicaemia, and infection of the central nervous system.
The emergence of drug resistant, including multi-drug resistant (MDR), bacterial isolates from clinical samples, foods, pharmaceuticals, as well as cosmetics, including eyecosmetics, is a cause of great concern. The bacterial isolates of S. aureus, Bacillus spp., Klebsiella spp., and P. aeruginosa isolated from cosmetic products (lotion and creams) showed resistance to one or more of the antibiotics: amoxycillin, augmentin, cotrimoxazole (COT), TC, NA, nitrofurantoin, CIP, GEN, ofloxacin and erythromycin [42]. The isolates of C. violaceum had sensitivity to GEN, AK, norfloxacin, COT, CM and TC, while resistance to AMP, cefazolin and ceftazidime, as has been reported by Jitmuang [43]. The B. cereus isolates from various sources showed resistance to AMP, cephalosporins, penicillin and trimehoprim, and sensitivity to aminoglycosides, CM, CIP, clindamycin, erythromycin, imipenem and VA, [44]. Banerjee et al. [45] reported that the most of the clinical B. cereus isolates had resistance to amoxyclav and cephalosporins. The bacterial strains isolated from different cosmetics including ‘kajal’ were identified as E. coli, Staphylococcus sp. and Bacillus sp., and the isolates were found resistant to one or more antibiotic tested such as CM, TC and SM [8]. Thus, the report on antibiotic resistance of eyecosmetic bacteria is meagre, and in the current study we have isolated different bacterial strains, of which Ps. aeruginosa, L. monocytogenes and C. violaceum were resistant to three or more antibiotics tested, while Bacillus spp. had sensitivity to all the test antibiotics. Regular surveillance of eye-cosmetic bacteria for antibiotic susceptibility is important and imperative, in order to control the infection caused by such strains in the community.
To the best of the authors’ awareness, this research has been the first to be carried out on antimicrobial susceptibility and MAR index determination for eye-cosmetic bacteria in our part of the globe. In this investigation, the two L. monocytogenes isolates, one from kohl and another from eye liner, had MAR index of 0.3; the B. cereus isolates had ‘zero’ MAR index. The MAR index was recorded as 0.4 for C. violaceum isolates from eye liner and kohl samples, while MAR index was calculated as 0.5 for mascara isolate of P. aeruginosa. Several earlier authors calculated the MAR index for bacteria isolated from different sources in order to evaluate the health risk of the environments, to provide the baseline information about the source of the bacterial contaminants and to identify the origin of resistance; the MAR indices >0.25 pose high risk source of contamination [19,46]. Tambekar et al. [47] reported that bacteria having MAR Index of >0.2 have originated from an environment where several antibiotics are in use. Subramani and Vignesh [48] determined MAR index of >0.2 for the bacterial strains tested, and reported that the isolates were transmitted from an environment of high antibiotic usage. Maloo et al. [49] reported ARI values of 0.03-0.07 for the test bacterial isolates including P. aeruginosa, and also recorded that 97% of the isolates were MDR with high MAR index (>0.2), suggesting the origin of the test isolates was of the high antibiotic usage. As has been reported by Oluyege et al. [50], the high level of MAR index (0.81-3.08), as compared to low risk value of 0.2 [19], might be the evidence of public health risk. As per the report of Chandran et al. [51], the MAR indices of 0.33-1 for the bacterial strains tested suggested the probable origin of such contamination from high risk source. Based upon the MAR index calculation, the current eye-cosmetic bacterial isolates (for which overall antibiotic resistance index was 0.055) have been categorized in to three: bacterial group having MAR index “zero” (Bacillus spp.), the group having MAR index of less than 0.3 (L. monocytogenes), and the group for which MAR index was ≥0.4 (C. violaceum and Ps. aeruginosa). Thus, findings of the current study suggest that P. aeruginosa originated from a very high risk source of contamination with increased number of antibiotic usage, while L. monocytogenes and C. violaceum from the sources of moderate to high risks. Since the above mentioned bacteria (L. monocytogenes, C. violaceum and P. aeruginosa) have the capacity to cause nosocomial and community acquired infection, use of such eye-cosmetics with bacterial contaminants might pose a serious threat to humans. Hence, public awareness on cosmetic safety as well as their prudent usage is strongly acclaimed on one side, and on the other maintenance of hygienic setting during production and packing, scientific study-based application of preservatives, phytomedicines and probiotics (since these are excellent antimicrobials) [52-55], as well as judicial use of antibiotics in such products is highly recommended.
13606
References
- Hugbo PG, Onyekweli AO, Igwe I (2003) Microbial contamination and preservative capacity of some brands of cosmetics. Trop J Pharma Res 2: 229-234.
- Gamal MAB, Abo Azza MM, AlGayeed AOA (2015) Sawan MS. Microbiological quality assessment of some brands of cosmetic creams sold within Alkhoms city, Libya. J Dental Med Sci 14: 60-65.
- Mohta A (2010) Kajal (Kohl) – A dangerous cosmetic. Oman J Ophthalmol 3: 100-101.
- Al-Aany LM, Sulyman SM, Muhammed MJ, et al. (2009) Isolation and identification of microbial flora in eye and study the effect of eye liner and contact lenses using on the microbial flora on it. Al-Anbar J for Pure Sci 3: 23-30.
- Campana R, Scesa C, Patrone V, Vittoria E, Baffone W (2006) Microbiological Study of cosmetic products during their use by consumers: health risk and efficacy of preservative systems. LettApplMicrobiol 43: 301-306.
- Orus P, Leranzo S (2005) Current trends in cosmetic microbiology. Int J Microbiol 8: 139- 142.
- Dawsen NL, Reinhardt DJ (1981) Microbial flora of in-use, display eye shadow tester and bacterial challenges of unused eye shadows. ApplEnviroMicrobiol 42: 297-302.
- Guleria A (2014) Isolation and identification of bacteria from different cosmetic samples and to check antimicrobial activity of antibiotics on bacteria isolated.Int J Scientific Res 3: 462-465.
- Abdelaziz AA, Ashour MSE, Hefni H, El-Tayeb OM (1989) Microbial contamination of cosmetics and personal care items in Egypt-eye shadows, mascaras and face creams. J Clin Pharmd Therapeutics 14: 21-28.
- Abdelaziz AA, Alkofahi A (1989) Microbiological profile of selected samples of "Al-Kohl" eye cosmetics in northern Jordanian provinces before and after use. Preventive Med 187: 244-253.
- Isa A, Elsie S, Nuhu OW (2014) Is topical kohl ophthalmic application associated with neonatal aseptic or bacterial conjunctivitis? Sub-Saharan Afr J Med 1: 138-41.
- Akrayi HFS (2012) Effect of some plant extracts on isolated bacteria from eyelids of natural eye liner users and eye cosmetics users. J ApplPharma Sci 2: 3-8.
- Baqer YM, Mohammed BB, Ali Obaid K, Ali Hlail J (2014) CFS of Lactobacillus: a natural agent against bacterial contamination of cosmetics tools. Int J Advanced Biol Res 4: 258-264.
- Das KK, KaziKaniz F, IfraTun N, Rashed N (2013) Prevalence of microorganisms in commonly used cosmetics samples in Dhaka metropolis. JPSI 2: 7-9.
- Holt JG (1984) Bergey’s Manual of Systematic Bacteriology, Williams and Wilkins, Baltimore.
- Forbes BA, Sahm DF, Weissfeld AS (2007) Bailey and Scott’s Diagnostic Microbiology. 12th Edition, Mosby (Elsevier), USA.
- Bauer AW, Kirby WMM, Skerris JC, Turuck M (1966) Antibiotic susceptibility testing by a standard single diffusion method. Am J Clin Pathol 45: 494-496.
- Clinical and Laboratory Standards Institute (CLSI): Performance standards for antimicrobial susceptibility testing (2011) 21st informational supplement M100S21. CLSI, Wayne, Pa.
- Krumperman PH (1983) Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol 46: 165-70.
- Abo-State MAM, Husseiny SHM, Helimish FA, Zickry ARA (2012) Contamination of eye drops with Bacillus species and evaluation of their virulence factors. World Appl Sci J 19: 847-855.
- Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK (2009) Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. SQU Med J 9: 184-195
- Lima-Bittencourt CI, Astolfi-Filho S, Chartone-Souza E, Santos FR, Nascimento AMA (2007) Analysis of Chromobacterium sp. natural isolates from different Brazilian ecosystems. BMC Microbiology 7: 58.
- Sivendra R, Tan SH (1977) Pathogenicity of nonpigmented cultures of Chromobacterium violaceum. J Clin Microbiol 5: 514-516.
- Kim MH, Lee HJ, Suh JT, Chang BS, Cho KS (2005) A Case of Chromobacterium infection after car accident in Korea. Yonsei Medical J 46: 700-702.
- Hills S (1946) The isolation of C. tetani from infected talcs. New Zealand Med J 45: 419-421.
- Wilson LA, Ahearn DG (1977) Pseudomonas-induced corneal ulcers associated with contaminated eye mascara. Asm J Ophthalmol 5: 112-119.
- Reid FR, Wood TO (1979) Pseudomonas cornealulcer.The causative role of contaminated eye cosmetics. Arc Opthalmol 97: 1640-1641.
- Neza E, Centini M (2016) Microbiologically contaminated and over-preserved cosmetic products according Rapex 2008-2014. Cosmetics 3: 3.
- Chang CY, Lee YT, Liu KS, Wang YL, Tsao SM (2007) Chromobacterium violaceum infection in Taiwan: a case report and literature review. J MicrobiolImmunol Infect 40: 272-275.
- Baker S, Campbell JI, Stabler R, Nguyen HVM, To DS, et al. (2008) Fatal wound infection caused by Chromobacterium violaceum in Ho Chi Minh City, Vietnam. J Clin Microbiol 46: 3853-3855.
- Slesak G, Douangdala P, Inthalad S, Silisouk J, Vongsouvath M, et al. (2009) Fatal Chromobacterium violaceum septicaemia in northern Laos, a modified oxidase test and post-mortem forensic family G6PD analysis. Annals Clin MicrobiolAntimicrob 8: 24.
- Berthold-Pluta A, Pluta A, Molska I, Dolega E (2014) Study on the survival of Bacillus cereus in media simulating the human stomach environment. Vet Med Practice 70: 437-441.
- Drobniewski FA (1993) Bacillus cereus and related species. Clin Microbiol Rev 6: 324-338.
- StenforsArnesen LP, Fagerlund A, Granum PE (2008) From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32: 579-606.
- Pitt TL, McClure J, Parker MD, Amezquita A, McClure PJ (2015) Bacillus cereus in personal care products: risk to consumers. Int J Cosmet Sci 37: 165-174.
- Streeter K, Katouli M (2016) Pseudomonas aeruginosa: A review of their pathogenesis and prevalence in clinical settings and the environment. Infect Epidemiol Med 2: 25-32.
- Eguchi H, Miyamoto T, Kuwahara T, Mitamura S, Mitamura Y (2013) Infectious conjunctivitis caused by Pseudomonas aeruginosa isolated from a bathroom. BMC Research Notes 6: 245.
- Green M, Apel A, Stapleton F (2008) Risk factors and causative organisms in microbial keratitis. Cornea 27: 22-27.
- Shen EP, Hsieh YT, Chu HS, Chang SC, Hu FR (2015) Correlation of Pseudomonas aeruginosa genotype with antibiotic susceptibility and clinical features of induced central keratitis. Invest Ophthalmol Vis Sci 56: 365-371.
- Jebur KS, Al-Hamadani AH, Jebur MS (2015) Evaluation of genetic study and bacterial culture for diagnosis of pseudomonal eye infections. CurrMicrobiolAppl Sci Int J 4: 348-356.
- Mierzejewski J, Kosek AW (2012) Microbes indicators of cosmetic preservation efficiency. Part I–Pseudomonas aeruginosa. Military Pharm Med 2: 32-40.
- Osungunna MO, Oluremi BB, Adetuyi A (2010) Bacteriological and antibiotic sensitivity patterns of bacterial isolates from creams and lotions hawked in Sagamu, Ogun State. Pak J Nutrition 9: 773-775.
- Jitmuang J (2008) Human Chromobacteriumviolaceum infection in Southeast Asia: case reports and literature review. Southeast Asian J Trop Med Public Health 39: 452-460.
- Turnbull PCB, Sirianni NM, LeBron CI, Samaan MN, Sutton FN, et al. (2004) MCIs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J Clin Microbiol 42: 3626-3634.
- Banerjee M, Nair GB, Ramamurthy T (2011) Phenotypic and genetic characterization of Bacillus cereus isolated from the acute diarrhoeal patients. Indian J Med Res 133: 88-95.
- Florea AB (2011) Antimicrobial susceptibility of Escherichia coli isolate from Aries river (Romania). Tom XVIII 1: 34-38.
- Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelval VK, Dudhane MN (2006) Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. African J Biotechnol 5: 1562-1565.
- Subramani S, Vignesh S (2012) MAR index study and MDR character analysis of a few golden Staph isolates. Asian J Pharma Life Sci 2: 151-154.
- Maloo A, Borade S, Dhawde R, Gajbhiye SN, Dastager SG (2014) Occurrence and distribution of multiple antibiotic-resistant bacteria of Enterobacteriaceae family in waters of Veraval coast, India. Environ Exp Biol 12: 43-50.
- Oluyege JO, Dada O, Oluyege AO, Olowomofe TO (2014) Multiple antibiotic resistance index of Escherichia coli isolated from drinking water sources in Ado-Ekiti, Nigeria. Experiment 28: 1896-1905.
- Chandran A, Hatha AAM, Varghese S, Sheeja KM (2007) Multiple antibiotic resistance profiles of various Escherichia coli serotypes isolated from Cochin Estuary. J Mar Atmos 3: 18-28.
- Mandal S, Mandal M (2015) Coriander (Coriandrumsativum L.) essential oil: chemistry and biological activity. Asian Pacific J Trop Biomed 5: 421-428.
- Halder D, Mandal S (2015) Probiotic potentiality of curd lactobacilli. Transl Biomed 6: 8.
- Halder D, Mandal S (2016) Antibacterial potentiality of commercially available probiotic lactobacilli and curd lactobacilli strains, alone and in combination, against human pathogenic bacteria. Transl Biomed 7: 2.
- Manilal A, Mama M, Gezmu T, Merdekios B, Ameya G, et al. (2016) An in vitro antibacterial and cytotoxic potentials of bioactive metabolites extracted from Padinatetrastromatica. Transl Biomed 7: 1.