Keywords
Western Ghats, Thirthahalli, Actinomycetes, Streptomyces, Biological activities
Introduction
Natural products produced from plants, animals and microbes have been used for several years. However, the scientific knowledge of natural products has been known over the last 100 years or so [1]. Actinomycetes are high G+C containing, filamentous, Gram positive bacteria that are widespread in nature. They are distributed in a variety of ecological habitats such as soil, fresh water, marine environment, plants (as endophytes) and others. They are involved in the decomposition of organic matter in soil, including lignin and other recalcitrant polymers, and can degrade agricultural and urban wastes [2,3,4]. The actinomycetes and their bioactive metabolites have shown to possess antimicrobial, cytotoxic, plant growth promotory, antiviral, antioxidant, insecticidal, antiprotozoal, anthelmintic, enzyme inhibitory, plant growth promoting and herbicidal agents [5-15]. They are known to play key role in bioremediation of dyes, petroleum products and pesticides and biosorption of heavy metals [16-20]. The bioactive metabolites from actinomycetes are also active against antibiotic resistant bacteria and plant pathogenic fungi [21-25].
Among actinomycetes, the genus Streptomyces is represented in nature by the largest number of species and varieties. These organisms differ in their morphology, physiology and biochemical characteristics. They have been distributed in every possible ecological niche. Species of the genus Streptomyces are dominating in soil in terms of number and the bioactive compounds what they produce. These organisms have been considered as potential sources of pharmaceutically and agriculturally important bioactive agents. They have provided 2/3rd of naturally occurring antibiotics, such as tetracyclines, aminoglysides, macrolides etc., discovered so far [22,26-30]. Western Ghats are one of the biodiversity hotspots in the world. These are grandly known for their diverse flora and fauna. However, a very few microbiological studies have been done in this region. Few studies have been carried out on the actinomycetes of Western Ghats of Karnataka and report antimicrobial, antioxidant, enzyme inhibitory, cytotoxic, anthelmintic, analgesic, anti-inflammatory and antipyretic activity [31-39]. The present study describes isolation and the biological activities viz., antibacterial, insecticidal, anthelmintic and antioxidant activity of a Streptomyces species SRDP-07 from a soil sample collected at Thirthahalli, Shivamogga (District), Karnataka (State), India.
Materials and Methods
Collection of soil sample
The soil sample was collected at Thirthahalli, Shivamogga (district), Karnataka (state), India during the month of July 2012. The soil was collected in a sterile plastic cover from a depth of 15cm, brought to the laboratory and dried at 40oC under aseptic conditions [35].
Isolation of actinomycetes
For isolation of actinomycetes, the soil sample was subjected to serial dilution followed by plating on Starch casein nitrate agar amended with antibiotic Fluconazole (to prevent fungal contamination). The plates were incubated aerobically at 30oC for 10 days. Colonies of actinomycetes were selected on the basis of typical colony morphology. The isolates were subcultured on Starch casein agar slants and maintained in refrigerator [35].
Primary screening for antibacterial potential of the actinomycetes isolates
Cross streak method was employed to screen antibacterial efficacy of the actinomycete isolates. The actinomycete isolates were streaked at the centre of the sterile Starch casein agar plates and the plates was incubated at 30°C for 5 days. After 5 days test bacteria were streaked perpendicular to the growth of the actinomycete isolates. The extent of growth inhibition of the test bacteria was observed after 24 hours of incubation. The absence of growth or a less dense growth of test bacteria near the actinomycete isolate was considered positive for production and secretion of antibacterial metabolite by the isolates [40]. One isolate (designated as isolate SRDP-07) displaying marked inhibition of test bacteria was selected for further identification and for assessing biological activities.
Characteristics of the isolate SRDP-07
The isolate SRDP-07 was further subjected for cultural, microscopic, staining and biochemical characteristics.
Cultural characteristics
The isolate SRDP-07 was grown on various media viz., Starch casein nitrate agar (SCA), Inorganic salt-starch agar (ISSA), Actinomycetes isolation agar (AIA) and Tryptone yeast extract agar (TYEA). The color of substrate and aerial mycelium was noted. The plates were observed for the production of diffusible pigments.
Microscopic characteristic
The cover slip method was followed to observe characteristic spore arrangement in isolate SRDP- 07. Thin blocks of SCA were cut and placed on sterile glass slides. The culture of SRDP-07 was inoculated all over the agar block surface using sterile inoculation loop, a cover slip was placed over the block, and the slide was placed in a sterile moist chamber and incubated until good growth of the isolate was observed. The cover slip was removed, placed on a drop of dilute crystal violet stain on a clean glass slide and observed under oil immersion objective in order to study the arrangement of spores [35].
Staining and biochemical characteristics
Staining techniques viz., Gram’s and Acid-fast staining and biochemical tests viz., starch hydrolysis, gelatin liquefaction, casein hydrolysis, catalse test, oxidase test, citrate test, cellulose hydrolysis, hydrogen sulfide (H2S) production test and sugar fermentation tests were performed for the isolate SRDP-07 [41,42].
Fermentation and extraction of metabolite from isolate SRDP-07
Sterile Starch casein broth containing Erlenmeyer flasks were inoculated with the spore supsenion of well sporulated actinomycete culture and the flasks were incubated aerobically at 28°C for 10 days. After incubation, the broth was aseptically filtered through sterilized Whattman No. 1 filter paper [35]. The culture filtrate was centrifuged and the supernatant was subjected solvent extraction in separation funnel and extracted using ethyl acetate. Equal volume (1:1) of supernatant and ethyl acetate were taken in a separation funnel and agitated for about 30 minutes. Solvent layer was separated and the supernatant was again extracted with ethyl acetate. The solvent layers were pooled and evaporated to dryness at 40°C [29]. The solvent extract was screened for biological activities.
Antibacterial activity of ethyl acetate extract of SRDP-07
The efficacy of ethyl acetate extract of SRDP-07 to inhibit bacteria was tested against two Gram positive bacteria viz., Staphylococcus aureus and Bacillus cereus and four Gram negative bacteria viz., Escherichia coli, Shigella flexneri, Klebsiella pneumoniae and Vibrio cholerae by Agar well diffusion method [43]. The test bacteria were inoculated into sterile nutrient broth (HiMedia, Mumbai) tubes and incubated for 24 hours at 37°C. The broth cultures of test bacteria were swabbed aseptically on sterile nutrient agar (HiMedia, Mumbai) plates with the help of sterile cotton swabs. Using a sterile cork borer, wells of 6mm diameter were punched in the inoculated plates and 100μl of ethyl acetate extract (5mg/ml of 10% DMSO), standard (Streptomycin, 1mg/ml) and DMSO (10%) were transferred into labelled wells. The plates were then incubated at 37oC for 24 hours and the zone of inhibition formed was measured. The experiment was repeated twice and the average value was recorded.
Insecticidal activity of ethyl acetate extract of SRDP-07
The insecticidal activity in terms of larvicidal effect of different concentrations (1 and 2mg/ml) of ethyl acetate extract was screened against II instar larvae of Aedes aegypti mosquito. Briefly, 20 larvae were transferred into beakers containing different concentrations of extract. A control was kept without adding extract. The larvicidal effect was determined by counting the number of dead larvae after 24 hours. Larvae that failed to move after probing with a needle in siphon or cervical region were identified as dead larvae. The experiment was repeated two times and average mortality (%) was noted [32].
Anthelmintic activity of ethyl acetate extract of SRDP-07
The anthelmintic effect of extract was screened against adult Indian earthworms (Pheretima posthuma). The worms were washed with normal saline (0.85%) to remove extraneous matter. In brief, 6 worms of equal size (6cm long) were transferred into normal saline containing standard (1%) and different concentrations of extract (1 and 2mg/ml of saline). The time taken for paralysis and death of worms was noted. Piperazine citrate was used as standard anthelmintic. Normal saline served as control. The experiment was repeated twice and average paralysis and death time was noted [31].
Antioxidant activity of ethyl acetate extract of SRDP-07
DPPH free radical scavenging activity
In order to determine radical scavenging efficacy of ethyl acetate extract, we have employed DPPH free radical scavenging assay [31]. In brief, 2ml of DPPH solution (0.002% in methanol) was mixed with 2ml of different concentrations (1- 100μg/ml) of extract and standard separately. The tubes were incubated in dark at room temperature for 30 minutes and the optical density was measured at 517 nm using UV-Vis spectrophotometer. The absorbance of the DPPH control was also noted. Ascorbic acid was used as reference standard. The scavenging activity of the extract was calculated using the formula: Scavenging activity (%) = [(A – B) / A] x 100, where A is absorbance of DPPH and B is absorbance of DPPH and extract/standard combination.
Ferric reducing activity
The reducing potential of ethyl acetate extract was determined by Ferric reducing assay. Here, different concentrations (1-100μg/ml) of extract and ascorbic acid (reference standard) in 1ml of methanol were mixed separately with 2.5ml of phosphate buffer (200 mM, pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The tubes were incubated in water bath for 20 minutes at 50oC, cooled rapidly and mixed with 2.5ml of 10% trichloroacetic acid and 0.5 ml of 0.1% ferric chloride. The amount of iron (II)-ferricyanide complex formed was determined by measuring the formation of Perl’s Prussian blue at 700 nm after 10 minutes. An increase in absorbance on increase in concentration indicates increased reducing power [31].
UV absorption of ethyl acetate extract of SRDP-07
The crude bioactive extract was dissolved in ethyl acetate solvent and subjected to UV absorption studies. The absorption spectrum of ethyl acetate extract was determined in the UV region (200- 400nm) by using a UV-visible spectrophotometer (Shimadzu UV 2554) [44].
Results
A total of 9 actinomycete isolates (SRDP-01 to SRDP-09) were recovered from the soil sample by serial dilution-plating method. All the isolates were subjected for assessment of antagonistic property against a panel of 6 bacteria by cross streak method. Absence of growth of test bacteria or reduced growth of test bacteria in the vicinity of the growth of actinomycetes was considered as positive for antibacterial activity. All the isolates showed inhibition of at least one of the test bacteria. Isolates SRDP-05 and SRDP-08 inhibited only one among six test bacteria. Prominent inhibitory activity was demonstrated by the isolate SRDP-07. Table 1 shows the extent of inhibition of test bacteria.
| Isolate |
Extent of inhibition of test bacteria |
| E.coli |
S.flexneri |
K.pneumoniae |
B.cereus |
V.cholerae |
S.aureus |
| SRDP-01 |
+++ |
++ |
++ |
+ |
++ |
+++ |
| SRDP-02 |
+ |
+ |
++ |
++ |
+ |
+++ |
| SRDP-03 |
+ |
+ |
+++ |
+ |
++ |
+ |
| SRDP-04 |
+ |
+ |
+ |
+ |
- |
+ |
| SRDP-05 |
- |
- |
+ |
- |
- |
- |
| SRDP-06 |
+++ |
+ |
+ |
+ |
+ |
+ |
| SRDP-07 |
+++ |
++++ |
++++ |
++ |
++++ |
++++ |
| SRDP-08 |
+ |
- |
- |
- |
- |
- |
| SRDP-09 |
+ |
+ |
- |
++ |
- |
- |
Table 1: Primary screening of actinomycete isolates for antibacterial activity
The growth characteristics of the isolate SRDP-07 was studied on four media viz., SCA, ISSA, AIA and TYEA. The color of both substrate and aerial mycelium was yellow on SCA and ISSA. On TYEA, the substrate and aerial mycelium was yellow and grey respectively. Both substrate and aerial mycelia were grey on AIA. Diffusible pigments were not observed in any of these media (Table 2).
| Media |
Substrate mycelium |
Aerial mycelium |
Diffusible pigment |
| SCA |
Yellow |
Yellow |
- |
| ISSA |
Yellow |
Yellow |
- |
| AIA |
Grey |
Grey |
- |
| TYEA |
Yellow |
Grey |
- |
Table 2: Cultural characteristics of isolate SRDP-07 on various media
The isolate SRDP-07 was Gram positive and nonacid fast. The spore arrangement was found to be closed spiral type. Biochemically, the isolate was found positive for amylase, cellulose, catalase and citrase. The isolate was not found to produce gelatinase, caseinase, oxidase and H2S. The isolate fermented glucose, fructose and galactose with acid production and maltose and lactose with alkali production. No gas production was observed (Table 3; Figure 1).
| Test |
Characteristic |
| Gram’s staining |
Gram positive |
| Acid fast staining |
Non-acid fast |
| Spore arrangement |
Closed spiral |
| Starch hydrolysis |
+ |
| Cellulose hydrolysis |
+ |
| Gelatin liquefaction |
- |
| Casein hydrolysis |
- |
| Catalase |
+ |
| Oxidase |
- |
| Citrate test |
+ |
| H2S production |
- |
| Fermentation |
Glucose- Acid Fructose- Acid Galactose- Acid Maltose- Alkali Lactose- Alkali |
Table 3: Microscopic, staining and biochemical characteristics of the isolate SRDP-07
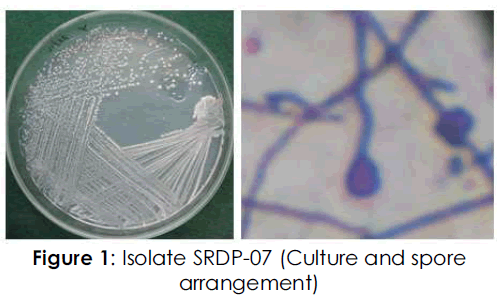
Figure 1: Isolate SRDP-07 (Culture and spore arrangement)
Ethyl acetate extract obtained after solvent extraction of culture filtrate was checked for antibacterial activity by Agar well diffusion method in which positive result was observed as the presence of zone of inhibition around the well. It was found that the extract was effective against all the test bacteria with zone of inhibition ranging from 1 cm to 2.4 cm. Susceptibility to extract was higher in case of Gram positive bacteria than Gram negative bacteria. Among the test bacteria, S. aureus and V. cholerae were highly susceptible Gram positive and Gram negative bacteria respectively. The extract was least effective against K. pneumoniae. Inhibition of test bacteria by standard antibiotic was higher than that of ethyl acetate extract. DMSO did not cause inhibition of test bacteria (Table 4). UV absorption maximum of ethyl acetate extract showed a single peak at 214nm.
| Test bacteria |
Zone of inhibition in cm |
| Extract |
Standard |
DMSO |
| E. coli |
1.2 |
2.8 |
0.0 |
| S. flexneri |
1.6 |
3.6 |
0.0 |
| K. pneumoniae |
1.0 |
3.4 |
0.0 |
| B. cereus |
2.3 |
3.8 |
0.0 |
| V. cholerae |
2.0 |
3.5 |
0.0 |
| S .aureus |
2.4 |
4.1 |
0.0 |
Table 4: Antibacterial activity of ethyl acetate extract of isolate SRDP-07
Ethyl acetate extract of isolate SRDP-07 was found to exhibit marked insecticidal activity against II instar larvae of A. aegypti. A dose dependent larvicidal effect was observed in this study. At 2mg/ml concentration, the mortality of larvae was 100% (Figure 2).
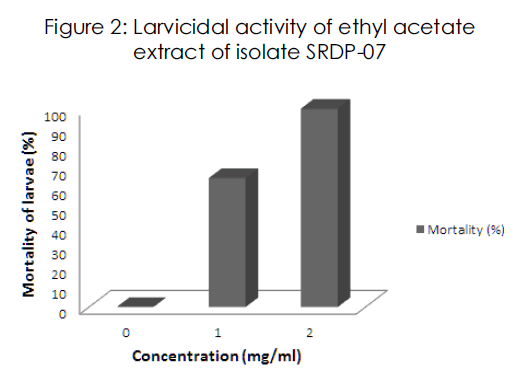
Figure 2: Larvicidal activity of ethyl acetate extract of isolate SRDP-07
Anthelmintic effect of ethyl acetate extract and piperazine citrate was determined on the basis of time taken for causing paralysis and death of adult Indian earthworms. The anthelmintic effect was observed as loss of motility and no response to external stimuli which eventually progressed to death of worms. The extract caused dose dependent paralysis and death of worms. Standard anthelmintic piperazine citrate exhibited stronger anthelmintic activity than that of ethyl acetate extract (Table 5).
Radical scavenging ability of different concentrations of ethyl acetate extract and ascorbic acid was evaluated using DPPH free radical assay. The extract exhibited antioxidant activity by scavenging DPPH* (free radical) and converting into DPPHH and the activity was found to be dose dependent. However, the scavenging potential of extract was found to be much lesser when compared with reference standard i.e., ascorbic acid. Lower concentrations of extract viz., 1 and 2.5μg/ml did not shown scavenging of free radicals (Figure 3).
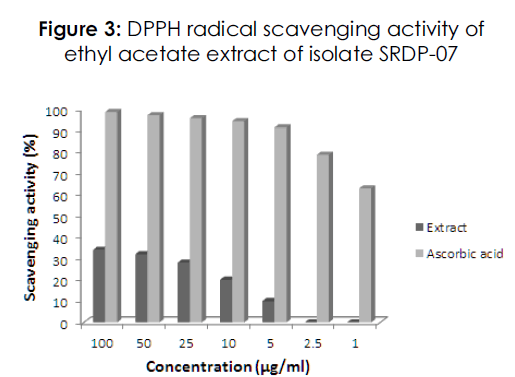
Figure 3: DPPH radical scavenging activity of ethyl acetate extract of isolate SRDP-07
The reducing potential of ethyl acetate extract and ascorbic acid was determined by employing ferric reducing assay in which the reduction of Fe3+ to Fe2+ was investigated in the presence of different concentrations of extract and ascorbic acid. The absorbance at 700nm was found to increase with the increase in concentration of extract indicating reducing potential of extract. The reducing potential of extract was lesser when compared with the reference standard (Figure 4).
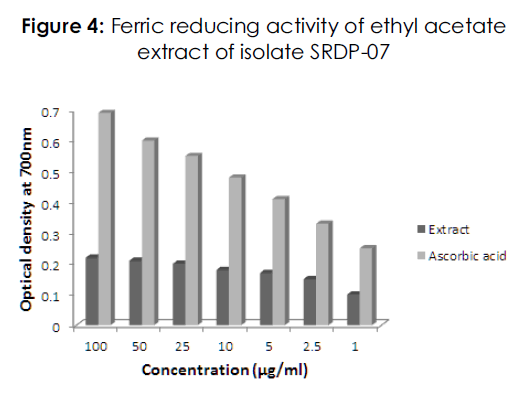
Figure 4: Ferric reducing activity of ethyl acetate extract of isolate SRDP-07
Discussion
Natural products are the most important source of new compounds having chemical diversity still unmatched by combinatorial chemistry approaches. These natural compounds have been used in the synthesis of several drugs. It is well known that microorganisms, in particular bacteria and fungi are virtually an unexhaustible source of natural compounds having several therapeutic applications [14,45,46]. Among microorganisms, the filamentous bacteria i.e., actinomycetes account for a significant fraction of microbial metabolites and Streptomyces is the most prolific genus producing wide range of bioactive metabolites among actinomycetes [47]. The genus Streptomyces comprises Gram positive, spore forming, aerobic actinomycetes having high DNA G-C% content (69~78 mol%) and classified in the family Streptomycetaceae on the basis of morphological and cell-wall chemotaxonomic characters. The members produce extensively branched substrate mycelium and aerial hyphae. With more than 500 validly described species and subspecies, the taxon currently contains the largest number of species in the domain Bacteria [27].
Soil is one of the rich reservoirs of microorganisms. Rhizosphere is the fraction of soil which is in vicinity to plant roots and is thought to be of great importance to plant health and soil fertility. The root exudates of plants stimulate the growth of microbial populations in soil and hence, microbial activity is greatest in rhizosphere region [48]. Actinomycetes forms a key part of soil microbiota. In general, Streptomyces species are saprophytic and are commonly associated with soils, where they significantly contribute to the turnover of complex biopolymers such as cellulose, lignin etc. and antibiotics [49]. It has been found that about 90% of soil actinomycetes are reported to be Streptomyces species [22,50]. In this study, we have isolated nine actinomycetes from the rhizosphere soil sample of Thirthahalli, Shivamogga, Karnataka. Preliminary screening for antibacterial activity, carried out by cross streak method, showed varied antagonistic potential of the isolates. Cross streak method is used to determine antagonstic potential of actinomycetes. It has become one of the routinely used methods and has been employed by several authors to study antimicrobial potential of actinomycete isolates [35,37,40,44,51,52]. One isolate, designated SRDP-07 displayed marked inhibition of test bacteria and hence, selected for further characterization to genus level and to determine biological activities.
Morphology plays an important role in distinguishing Streptomyces from other sporing actinomycetes and in the characterization of Streptomycete species. The life cycle of a Streptomycete provides 3 features for microscopic characterization viz., vegetative mycelium, aerial mycelium baring chains of spores and the characteristics of spores themselves. The latter two features produce most diagnostic information [53,54]. Information on cultural features and characteristic spore arrangement together with biochemical properties assists classification of actinomycetes as members of the genus Streptomyces. Saadoun et al. [55] recovered nine different isolates of aquatic actinomycetes and identified them as Streptomyces spp. on the basis of morphological and cultural characteristics. Singh et al. [56] isolated two strains of Streptomyces species and identified them as Streptomyces albovinaceous based on cellular morphology and physiology. In a study by Savic et al. [57], morphological and phenotypic properties of actinomycete isolate MS405T were consistent with its classification as a Streptomyces strain. Similarly, other studies by Rifaat et al. [58], Sahin et al. [59] and Moncheva et al. [60] have assigned the certain actinomycetes strains under the genus Streptomyces on the basis of characteristics such as cultural, microscopic and biochemical features. In the present study, the cultural and microscopic characteristics of the isolate SRDP-07 were consistent with its classification as a member of the genus Streptomyces.
Infectious diseases are caused by a number of bacteria, viruses, parasites and fungi which devastated mankind before the development of chemotherapeutic agents mainly antibiotics. The discovery of antibiotics and their subsequent use has eradicated a lot of infections. However, traditional antibacterial therapy using antibiotics from microbial sources or their synthetic analogues is facing problems due to development of resistance in existing agents to antimicrobials. Staphylococcus aureus is one of the first pathogens that became resistant to almost all known antibiotics posing a global threat. Vancomycin resistant enterococci, multidrug resistant tuberculosis, antibiotic resistant E. coli, P. aeruginosa are among other antibiotic resistant bacteria against which most of the antibiotics are not effective. Moreover, these bacteria have the tendency to transmit the resistance gene and this has become a serious issue in the field of medicine [61-64]. The need for new antibiotics is increasing day by day due to the development and spread of antibioticresistant pathogens which cause life-threatening infections and patient sensitivity [49,65]. Actinomycetes have provided and continue to provide a number of antibiotics that are clinically relevant in the treatment of various diseases. They have the capability to produce a variety of compounds having diverse pharmacological activities [66]. Actinomycetes have shown to produce metabolites that are active against antibiotic resistant bacteria [24,67]. In the present study, the ethyl acetate extract of SRDP-07 showed marked inhibition of test bacteria. It was found that Gram positive bacteria have shown to be highly susceptible than Gram negative bacteria. Similar results were observed in the earlier studies [68-71]. The low antibacterial activity of ethyl acetate extract against the gram negative bacteria could be ascribed to the presence of an outer membrane that possess hydrophilic polysaccharides chains and forms an additional barrier for extract as well as antibiotics [72,73]. UV spectral studies of ethyl acetate extract of isolate SRDP-07 showed a single peak with absorbance maximum at 214nm. Similar absorption band was observed in an early study by Ezra et al. [74] for coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp. (MSU-2110).
Mosquitoes have a predominant role in the transmission of several diseases such as malaria, yellow fever, dengue fever, filariasis, and others. These mosquitoes spread more diseases than any other group of arthropods and also cause allergic responses that include local skin and systemic reactions [75,76,77]. Aedes aegypti is responsible for transmission of one of the serious arboviral diseases dengue. The control measures taken against arthropod-borne diseases target larval stages and adult mosquitoes. Control of the larvae of the mosquito is one of the widely employed strategies and is frequently done by the application of synthetic insecticides and insect growth regulators. However, the raising cost, possible toxicity hazards and insects showing resistance highlighted search for the development of alternatives for mosquito control [75,78]. Actinomycetes are shown to be promising sources of bioactive metabolites displaying potent insecticidal activity [32,35,79-82]. In the present study, we determined insecticidal activity of ethyl acetate extract of SRDP-07 against 2nd instar larvae of A. aegypti. The extract displayed a dose dependent mortality of larvae. Similar results have been noticed in earlier studies. The butanol extracts of two Streptomyces species from Agumbe region produced dose dependent mortality of 2nd instar larvae of A. aegypti [32]. In another study, the crude extract of a Streptomyces species isolated from Agumbe region of Western ghats of Karnataka displayed a dose dependent mortality of 3rd instar larvae of A. aegypti [35].
Helminthic infections are one of the major diseases worldwide in particular tropical countries. It is the major cause of morbidity and is often fatal in extreme conditions. Parasitic worms also infect livestock and crops, affecting food production resulting in low economy. Many factors influence susceptibility of an individual such as lack of sanitation and supply of pure water together with poverty and illiteracy. Helminthic infections contribute to the prevalence of malnutrition, anemia, eosinophilia, and pneumonia. Anthelmintic drugs that expel parasitic worms from the body have some major drawbacks such as resistance development in gastro-intestinal helminthes, high cost, adverse effects etc. This situation led to the discovery and development of anthelmintic agents from natural sources [83-86]. Actinomycetes have shown to possess marked anthelmintic activity in terms of inhibition of plant and animal parasites [12,31,87,88,89]. In this study, the anthelmintic activity of ethyl acetate extract of SRDP-07 was determined using adult Indian earthworms due to their ready availability and anatomical and physiological resemblance to the human intestinal roundworm parasite [84,90]. The standard anthelmintic piperazine citrate showed higher activity than the extract. The predominant effect of piperazine citrate on the worm is to cause a flaccid paralysis that result in expulsion of the worm [83,84]. The extract of SRDP-07 not only demonstrated this property but also killed the worms, however, the time taken for this was much higher when compared with standard. In an earlier study, Kekuda et al. [31] showed dose dependent mortality and death of earthworms by butanol extracts of two Streptomyces species isolated from western ghat soil of Agumbe, Karnataka.
A well maintained balance exists between antioxidant defence mechanisms and generation of free radicals in a normal healthy individual. During oxidative stress, this balance, however, shift towards the excessive production of free radicals or deficit in antioxidant defence mechanism. This oxidative stress is implicated in over hundreds of pathophysiological conditions such as diabetes, cardiovascular diseases, neurological disorders, cancer, aging etc. Antioxidant is a substance that significantly inhibits or delay oxidative processes. Endogenous antioxidants such as ascorbic acid, tocopherols, glutathione, uric acid, thiols etc., and antioxidant enzymes such as superoxide dismutase and catalase protect the body against oxidative stress. However, in pathophysiological conditions, there is additional requirement for antioxidants [91-95]. Strong restrictions have been placed on the use of synthetic antioxidants such as BHT, BHA, gallates due to their suspected carcinogenic potential [96]. This led to an increasing interest in natural products having antioxidant properties. Actinomycetes have been shown to be promising sources of antioxidants. A number of studies have been carried out on the antioxidant efficacy of extracts and purified compounds from actinomycetes [14,31,97-102].
DPPH free radical scavenging assay is one of the widely used protocol to arrive the radical scavenging ability of compounds. DPPH is a stable organic free radical having the absorption maximum around 515-528nm. On accepting an electron or hydrogen atom it becomes a stable diamagnetic molecule. The effect of antioxidants on scavenging DPPH radical is due to their hydrogen donating ability. In this assay, the compounds (antioxidants) reduce the purple colored DPPH radical to a yellow colored compound diphenylpicrylhydrazine and the extent of reaction depends on the hydrogen donating ability of the antioxidants [103,104,105]. In the present study, the absorption of DPPH in the presence of various concentrations of ethyl acetate extract of SRDP-07 was measured at 517nm. It was observed that the radical scavenging activities of the extract increased with increasing concentrations. Although the scavenging abilities of extract were much lesser than that of ascorbic acid, it was evident that the extract showed hydrogen donating ability and the extract could serve as free radical scavengers, acting possibly as primary antioxidants [104]. The results obtained are in justification with earlier studies which also reveal weaker scavenging activity of extracts [31,36,38].
Ferric reducing assay is one of the commonly used antioxidant assays which measures total antioxidant activity. The assay is performed in order to measure the reducing power of the compounds. In the present study, we investigated the Fe+3/Fe+2transformation in the presence of ethyl acetate extract of SRDP-07. In this assay, the reductants (antioxidants) would cause the reduction of Fe+3 to Fe+2 by donating an electron. The amount of Fe+2complex formed can be monitored by measuring the formation of Perl’s Prussian blue at 700nm. Increasing absorbance at 700nm indicates an increase in reductive ability [104]. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity [106]. In our study, the reducing power of the extract also increased with the increase of its concentration. However, the reducing potential of extract was lesser when compared with reference standard. Although the extract was found to possess less reducing power, it is evident that the extract possesses reductive potential and could serve as electron donors, terminating the radical chain reactions [104]. Similar results have been observed in previous studies of Kekuda et al. [31] and Manasa et al. [36] where the actinomycete extracts have displayed weaker reducing potential.
Conclusion
In the present study, we screened the bioefficacies of an actinomycete isolate recovered from a soil sample collected from Western Ghat region Karnataka, India. One isolate Streptomyces species SRDP-07 showed good antibacterial and insecticidal activity. The results of the present study highlighted that the soils of Thirthahalli are reservoirs of potent actinomycetes and hence further screening can be fruitful for isolation of bioactive actinomycetes which might be exploited in industries for production of novel metabolites.
Acknowledgements
The authors express thanks to Head, Department of Microbiology, Principal, SRNMN College of Applied Sciences, Shivamogga and NES, Shivamogga for giving support to conduct work.
7282
References
- Zerikly M, Challis GL. Strategies for the discovery of new natural products by genome mining. ChemBiochem 2009; 10(4): 625-633
- Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Applied and Environmental Microbiology 1997; 63(8): 3233-3241
- Bunyoo C, Duangmal K, Nuntagij A, Thamchaipenet A. Characterisation of endophytic actinomycetes isolated from wattle trees (Acacia auriculiformis A. Cunn. ex Benth.) in Thailand. Thai Journal of Genetics 2009; 2(2): 155–163
- Gonzalez-Franco AC, Robles-Hernandez L, Nuñez-Barrios A, Strap JL, Crawford DL. Molecular and cultural analysis of seasonal actinomycetes in soils from Artemisia tridentata habitat. International Journal of Experimental Botany 2009; 78: 83-90
- Yokomizo K, Miyamoto Y, Nagao K, Kumagae E, Habib SE, Suzuki K, Harada S, Uyeda M. Fattiviracin A1, a novel antiviral agent produced by Streptomyces microflavus strain No. 2445. II. Biological properties. Journal of Antibiotics 1998; 51(11): 1035-1039
- Yang SW, Chan TM, Terracciano J, Patel R, Loebenberg D, Chen G, Patel M, Gullo V, Pramanik B, Chu M. New antibiotic Sch 725424 and its dehydration product Sch 725428 from Kitasatospora sp. Journal of Antibiotics. 2005; 58(3): 192-195.
- Gorajana A, Krada BVVSN, Peela S, Jangam P, Vinjamuri S, Poluri E, Zeeck A. 1-Hydroxy-1- norresistomycin, a new cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Journal of Antibiotics 2005; 58(8): 526-529.
- Sousa CS, Soares ACF, Garrido MS. Characterization of Streptomycetes with potential to promote plant growth and biocontrol. Scientia Agricola 2008; 65(1): 50-55
- Herbicidal agents from actinomycetes against selected crop plants and weeds. Natural Product Research 2010; 24(6): 521-529
- Khamna S, Yokota A, Peberdy JF, Lumyong S. Indole-3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. EurAsian Journal of BioSciences 2010; 4: 23-32
- Pimentel-Elardo SM, Kozytska S, Bugni TS, Ireland CM, Moll H, Hentschel U. Anti-parasitic compounds from Streptomyces sp. strains isolated from Mediterrranean sponges. Marine Drugs2010; 8:373-380.
- Ruanpanum P, Laatsch H, Tangchitesomkid N, Lumyong S. Nematicidal activity of fervenulin isolated from a nematicidal actinomycete, Streptomyces sp. CMU-MH21, on Meloidogyyne incognita. World Journal of Microbiology Biotechnology 2011; 27: 1373-1380.
- Ababutain IM, Aziz ZKA, Al-Meshhen NA. Lincomycin antibiotic biosynthesis produced by Streptomyces sp. isolated from Saudi Arabia soil: I - Taxonomical, antimicrobial and insecticidal studies on the producing organisms. Canadian Journal of Pure and Applied Sciences 2012; 6(1): 1739-1748
- Kumar S, Krishnan K. Cytotoxicity and antioxidant activity of 5-(2,4- dimethylbenzyl)pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi Journal of Biological Sciences 2012; 19: 81-86
- Alam M, Dharni S, Abdul-Khaliq, Srivastava SK, Samad A, Gupta MK. A promising strain of Streptomyces sp. with agricultural traits for growth promotion and disease management. Indian Journal of Experimental Biology 2012; 50: 559-568
- De Schrijver A, De Mot R. Degradation of pesticides by actinomycetes. Critical Reviews in Microbiology 1999; 25(2): 85-119
- Li P, Guo S, Sun T, Tai P, Zhang C, Bai Y, Sun Q, Sheng P. Bio-remediation techniques of crude oil contaminated soils. Ying Yong Sheng Tai Xue Bao 2002; 13(11): 1455-1458
- Rho J, Kim J. Heavy Metal Biosorption and its Significance to Metal Tolerance of Streptomycetes. The Journal of Microbiology 2002; 40(1): 51-54
- El-Sersy NA, Abou-Elela GM, Hassan SW, Abd- Elnaby H. Bioremediation of acid fast red dye by Streptomyces globosus under static and shake conditions. African Journal of Biotechnology 2011; 10(17): 3467-3474
- Sanjenbam P, Kumar S, Krishnan K. Biosorption of mercury and lead by aqueous Streptomyces VITSVK9 sp. isolated from marine sediments from the bay of Bengal, India. Frontiers of Chemical Science and Engineering 2012; 6(2): 198-202
- Shekhar N, Bhattacharya D, Kumar D, Gupta RK. Biocontrol of wood-rotting fungi with Streptomyces violaceusniger XL-2. Canadian Journal of Microbiology 2006; 52(9): 805-808
- Narayana KJP, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y, Krishna PSJ. Biological activity of Phenylpropionic acid isolated from a terrestrial Streptomycetes. Polish Journal of Microbiology 2007; 56(3): 191-197
- Mcarthur KA. Mitchell SS, Tsueng G, Rheingold A, White DJ, Grodberg J, Lam KS, Barbara CMP. Lynamicins A-E, chlorinated bisindole Pyrrole antibiotics from a novel marine actinomycete. Journal of Natural Products 2008; 71: 1732-1737.
- Peoples AJ, Zhang Q, Millett WP, Rothfeder MT, Pescatore BC, Madden AA, Ling LL, Moore CM. Neocitreamicins I and II, Novel antibiotics with activity against methicillin resistant Staphylococcus aureus and vancomycin resistant enterococci. The Journal of Antibiotics 2008; 61(7): 457-463
- Ningthoujam DS, Sanasam S, Tamreihao K, Nimaichand S. Antagonistic activities of local actinomycete isolates against rice fungal pathogens. African Journal of Microbiology Research 2009; 3(11): 737-742
- Ramakrishnan J, Shunmugasundaram M, Narayana M. Streptomyces sp. SCBT isolated from rhizosphere soil of medicinal plants is antagonistic to pathogenic bacteria. Iranian Journal of Biotechnology 2009; 7(2): 75-81
- Ripa FA, Nikkon F, Zaman S, Khondkar P. Optimal Conditions for Antimicrobial Metabolites Production from a New Streptomyces sp. RUPA- 08PR Isolated from Bangladeshi Soil. Mycobiology 2009; 37(3): 211-214
- Gopal M, Mishra E, Das SK, Chatterjee SC, Sangle UR, Bambawale OM. Growth inhibition of different pathogenic fungi by Actinomycetes and its identification by 16S rRNA. Indian Phytopathology 2010; 63(2): 137-140
- Alimuddin, Widada J, Asmara W, Mustofa. Antifungal production of a strain of Actinomycetes spp isolated from the rhizosphere of Cajuput plant: Selection and detection of exhibiting activity against tested fungi. Indonesian Journal of Biotechnology 2011; 16(1): 1-10
- Rahman MA, Islam MAU. Molecular Characterization of Actinomycin D producing Streptomyces strain isolated from soil Samples. Bangladesh Pharmaceutical Journal 2012; 15(2): 113-117
- Kekuda PTR, Shobha KS, Onkarappa R. Studies on antioxidant and anthelmintic activity of two Streptomyces species isolated from Western Ghat soil of Agumbe, Karnataka. Journal of Pharmacy Research 2010; 3(1): 26-29
- Kekuda PTR, Shobha KS, Onkarappa R. Potent insecticidal activity of two Streptomyces species isolated from the soils of Western ghats of Agumbe, Karnataka. Journal of Natural Pharmaceuticals 2010; 1(1): 30-32
- Shobha K.S, Onkarappa R. In vitro susceptibility of C. albicans and C. neoformens to potential metabolites from Streptomycetes. Indian Journal of Microbiology 2011; 51(4): 445-449
- Kekuda PTR, Shobha KS, Onkarappa R. Pancreatic lipase Inhibitory and cytotoxic potential of a Streptomyces species isolated from Western Ghat soil, Agumbe, Karnataka, India. International Journal of Pharmaceutical and Biological Archives 2011; 2(3): 932-937
- Kekuda PTR, Shobha KS, Onkarappa R, Gautham SA, Raghavendra HL. Screening Biological Activities of a Streptomyces Species Isolated from soil of Agumbe, Karnataka, India. International Journal of Drug Development and Research 2012; 4(3): 104-114
- Manasa M, Poornima G, Abhipsa V, Rekha C, Kekuda PTR, Onkarappa R, Mukunda S. Antimicrobial and antioxidant potential of Streptomyces sp. RAMPP-065 isolated from Kudremukh soil, Karnataka, India. Science, Technology and Arts Research Journal 2012; 1(3): 39-44
- Gautham SA, Shobha KS, Onkarappa R, Kekuda TRP. Isolation, characterization and antimicrobial potential of Streptomyces species from Western Ghats of Karnataka, India. Research Journal of Pharmacy and Technology 2012; 5(2): 233-238
- Gautham SA, Onkarappa R. In vitro antioxidant activity of metabolite from Streptomyces fradiae strain GOS1. International Journal of Drug Development and Research 2013; 5(1): 235-244
- Gautham SA, Onkarappa R. Pharmacological activities of metabolite from Streptomyces fradiae strain GOS1. International Journal of Chemical Sciences 2013; 11(1): 583-590
- Dasari VRRK, Nikku MY, Donthireddy SRR. Screening of Antagonistic Marine Actinomycetes: Optimization of Process Parameters for the Production of Novel Antibiotic by Amycolatopsis alba var. nov. DVR D4. Journal of Microbial and Biochemical Technology 2011, 3:5
- Aneja KR. Experiments in Microbiology, Plant pathology, Tissue culture and Mushroom cultivation. 2nd Edition. Wishwa Prakashan, New Delhi, 1996
- Florencio C, Couri S, Farinas CS. Correlation between Agar Plate Screening and Solid-State Fermentation for the Prediction of Cellulase Production by Trichoderma Strains. Enzyme Research 2012, Volume 2012, Article ID 793708, 7 pages, doi: 10.1155/2012/793708
- Kekuda PTR, Raghavendra HL, Swathi D, Venugopal TM, Vinayaka KS. Antifungal and cytotoxic activity of Everniastrum cirrhatum (Fr.) Hale. Chiang Mai Journal of Science 2012; 39(1): 76-83
- Sahin N, Ugur A. Investigation of the antimicrobial activity of some Streptomyces isolates. Turkish Journal of Biology 2003; 27: 79-84
- El-Naggar MY, El-Assar SA, Abdul-Gawad SM. Meroparamycin production by newly isolated Streptomyces sp. strain MAR01: Taxanomy, fermantation, purfication and structural elucidation. The Journal of Microbiology 2006; 44(4): 432-438.
- Engelhardt K, Degnes KF, Kemmler M, Bredholt H, Fjaervik E, Klinkenberg G, Sletta H, Ellingsen TE, Zotchey SB. Production of a new thiopeptide antibiotic, TP-1161, by a Marine Nocardiopsis species. Applied and Environmental Microbiology 2010; 76(15): 4969-4976.
- Busti E, Monciardini P, Cavaletti L, Bamonte R, Lazzarini A, Sosio M, Donadio S. Antibiotic- producing ability by representatives of a newly discovered lineage of actinomycetes. Microbiology 2006; 152: 675–683
- Iznaga Y, Lemus M, Gonzalez L, Garmendia L, Nadal L, Vallin C. Antifungal activity of actinomycetes from Cuban soils. Phytotherapy Research 2004; 18: 4494-496
- Islam MR, Jeong YT, Ryu YJ, Song CH, Lee YS. Isolation, identification and optimal culture conditions of Streptomyces albidoflavus C247 producing antifungal agents against Rhizoctonia solani AG2-2. Mycobiology 2009; 37(2): 114-120
- Smaoui S, Mathieu F, Ben-Fguira LF, Merlina G, Mellouli L. Taxonomy and antimicrobial activities of a new Streptomyces sp. TN17 isolated in the soil from an oasis in Tunis. Archives of Biological Sciences 2011; 63(4): 1047-1056
- Haque SK, Sen SK, Pal SC. Antimicrobial spectra and toxicity of antibiotics From Streptomyces antibioticus SR 15-4. Indian Journal of Microbiology 1996; 36: 113-114
- Nanjwade B, Chandrashekhara S, Ali M, Goudanavar PS, Manvi FV. Isolation and morphological characterization of antibiotic producing actinomycetes. Tropical Journal of Pharmaceutical Research 2010; 9(3): 231-236
- Anderson AS, Wellington EMH. The taxonomy of Streptomyces and related genera. International Journal of Systematic and Evolutionary Microbiology 2001; 51: 797-814
- Taddei A, Rodriguez MJ, Marquez-Vilchez E, Castelli C. Isolation and identification of Streptomyces spp. From Venezuelan soils: Morphological and biochemical studies. I. Microbiological Research 2006; 161: 222-231
- Saadoun I, Hameed KM, Moussauui A. Characterization and analysis of antibiotic activity of some aquatic actinomycetes. Microbios. 1999; 99: 173-179
- Singh SK, Gurusiddaiah S, Whalen JW. Treponemycin, a nitrile antibiotic active against Treponema hyodysenteriae. Antimicrobial Agents and Chemotherapy 1985; 27(2): 239-245
- Savic M, Bratic I, Vasiljevic B. Streptomyces durmitorensis sp. nov., a producer of an FK506- like immunosuppressant. International Journal of Systematic and Evolutionary Microbiology 2007; 57: 2119-2124
- Rifaat HM, Awad AH, Gebreel HM. Taxonomic characterization of Actinobacteria isolated from the atmosphere surrounding Chamomile plants. Applied Ecology and Environmental Research 2004; 2(2): 45-51
- Sahin N, Ozturk E, Isik K, Karlptas E, Ozkanca R. Selective isolation and numerical classification of novel thermophilic Streptomycetes. Turkish Journal of Biology 2001; 26: 13-24
- Moncheva P, Tishkov S, Dimitrova N, Chipeva V, Antonova-Nikolova S, Bogatzevska N. Characteristics of soil actinomycetes from Antarctica. Journal of Culture Collections 2000- 2002; 3: 3-14
- Ojala T, Remes S, Haansuu P, Vuorela H, Hiltunen R, Haahtela K, Vuorela P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. Journal of Ethnopharmacology 2000; 73: 299-305
- Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008; 15: 639-652
- Davies J, Davies D. Origins and evolutions of antibiotic resistance. Microbiology and Molecular Biology Reviews 2010; 74(3): 417-433
- Wright GD. Q&A: Antibiotic resistance: where does it come from and what we can do about it? BMC Biology 2010; 8: 123
- Radhakrishnan M, Balagurunathan R, Selvakumar N, Doble M, Kumar V. Bioprospecting of marine derived actinomycetes with special reference to antimycobacterial activity. Indian Journal of Geo-Marine Sciences 2011; 40(3): 407-410
- Jain PK, Jain PC. Isolation, characterization and antifungal activity of Streptomyces sampsonii GS 1322. Indian Journal of Experimental Biology 2007; 45: 203-206
- Zhang X, Ren K, Zhang L. Screening and preliminary identification of medicinal plants endophytic actinomycetes used for inhibiting penicillin-resistant Staphylococcus aureus. International Journal of Biology 2012; 4(2): 119- 124
- Hassan MA, El-Naggar MY, Said WY. Physiological factors affecting the production of an antimicrobial substance by Streptomyces violatus in batch cultures. Egyptian Journal of Biology 2001; 3: 1-10
- Anansiriwattana W, Tanasupawat S, Amnuoypol S, Suwanborirux K. Identification and antimicrobial activities of actinomycetes from soils in Samed Island, and geldamycin from strain PC4-3. Thai Journal of Pharmaceutical Sciences 2006; 30: 49-56
- Valli S, Suvathi SS, Aysha OS, Nirmala P, Kumar VP, Reena A. Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pacific Journal of Tropical Biomedicine 2012; 2(6): 469-473
- Gunda MM, Charya MAS. Physiological factors influencing the production of antibacterial substance by freshwater actinobacteria. Journal of Recent Advances in Applied Sciences 2013; 28: 55-62
- Lodhia MH, Bhatt KR, Thaker VS. Antibacterial activity of essential oils from Palmarosa, Evening Primrose, Lavender and Tuberose. Indian Journal of Pharmaceutical Sciences 2009; 71(2): 134-136
- Nalubega R, Kabasa JD, Olila D, Kateregga J. Evaluation of Antibacterial Activity of Selected Ethnomedicinal Plants for Poultry in Masaka District, Uganda. Research Journal of Pharmacology 2011; 5(2): 18-21
- Ezra D, Castillo UF, Strobel GA, Hess WM, Porter H, Jensen JB, Condron MAM, Teplow DB, Sears J, Maranta M, Hunter M, Weber B, Yaver D. Coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp. (MSU-2110) endophytic on Monstera sp. Microbiology 2004; 150: 785-793
- Cheng S, Huang C, Chen W, Kuo Y, Chang S. Larvicidal activity of tectoquinone isolated from red heartwood-type Cryptomeria japonica against two mosquito species. Bioresource Technology 2008; 99: 3617-3622
- Cheng S, Huang C, Chen Y, Yu J, Chen W, Chang S. Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species. Bioresource Technology 2009; 100: 452-456
- Bagavan A, Rahuman AA. Evaluation of larvicidal activity of medicinal plant extracts against three mosquito vectors. Asian Pacific Journal of Tropical Biomedicine 2010; 4(1): 29-34
- Kaushik R, Saini P. Screening of some semi-arid region plants for larvicidal activity against Aedes aegypti mosquitoes. Journal of Vector Borne Diseases 2009; 46: 244-246
- Lewer P, Chapin EL, Graupner PR, Gilbert JR, Peacock C. Tartrolone C. A novel insecticidal macrolide produced by Streptomyces sp: CP1130. Journal of Natural Products 2003; 66: 143-145
- Xiong L, Li J, Kong F. Streptomyces sp. 173, an insecticidal microorganism from marine. Letters in Applied Microbiology 2004; 38: 32-37
- Gadelhak GG, El-Tarabily KA, Al-Kaabi FK. Insect control using chitinolytic soil actinomycetes as biocontrol agents. International Journal of Agriculture and Biology 2005; 7: 627-633
- Liu H, Qin S, Wang Y, Li W, Zhang J. Insecticidal action of Quinomycin A from Streptomyces sp. KN-0647, isolated from a forest soil. World Journal of Microbiology and Biotechnology 2008; 24: 2243-2248
- Mali RG, Wadekar RR. In vitro anthelmintic activity of Baliospermum montanum Muell. Arg roots. Indian Journal of Pharmaceutical Sciences 2008; 70(1): 131-133
- Kumar ABS, Lakshman K, Jayaveera KN, Nandeesh R, Manoj B, Ranganayakulu D. Comparative in vitro anthelmintic activity of three plants from the Amaranthaceae family. Achieves of Biological Sciences 2010; 62(1): 185- 189
- Das SS, Dey M, Ghosh AK. Determination of anthelmintic activity of the leaf and bark extract of Tamarindus indica Linn. Indian Journal of Pharmaceutical Sciences 2011; 73(1): 104-107
- Mehta DK, Das R, Bhandari A. In-vitro anthelmintic activity of seeds of Zanthoxylum armatum DC. against Pheretima posthuma. International Journal of Green Pharmacy 2012; 6(1): 26-28
- Burg RW, Miller Bm, Barker EE, Birnbaum J, Currie SA, Hartman R, Kong Y, Monaghan RL, Olson G, Putter I, Tunac JB, Wallick H, Stapley EO, Oiwa R, Omura S. Avermectins, New Family of Potent Anthelmintic Agents: Producing Organism and Fermentation. Antimicrobial agents and Chemotherapy 1979; 15(3): 361-367
- Haber CL, Heckaman CL, Li GP, Thompson DP, Whaley HA, Wiley VH. Development of a mechanism of action-based screen for anthelmintic microbial metabolites with avermectin like activity and isolation of Milbemycin producing Streptomyces strains. Antimicrobial agents and Chemotherapy 1991; 35(9): 1811-1817
- Al-Doori M, Al-Tae AA, Jalil S, Hassan SA. Larvicidal activity of actinomycete isolate against Toxocara canis. Folia Parasitologica 1991; 38: 379-382
- Muhammad N, Saeed M, Khan H, Qayum M, Barkatullah, Badshah A. Evaluation of Viola betonicifolia for anthelmintic activity. African Journal of Pharmacy and Pharmacology 2012; 6(10): 698-701
- Yen G, Duh P, Su H. Antioxidant properties of lotus seed and its effect on DNA damage in human lymphocytes. Food Chemistry 2005; 89: 379-385
- Elmastas M, Gulcin I, Isildak O, Kufrevioglu OI, Ibaoglu K, Aboul-Enein HY. Radical scavenging activity and antioxidant capacity of Bay leaf extracts. Journal of Iranian Chemical Society 2006; 3(3): 258-266
- Dixit P, Ghaskadbi S, Mohan H, Devasagayam TPA. Antioxidant properties of germinated fenugreek seeds. Phytotherapy Research 2005; 19: 977-983
- Chatterjee S, Poduval TB, Tilak JC, Devasagayam TPA. A modified, economic, sensitive method for measuring total antioxidant capacities of human plasma and natural compounds using Indian saffron (Crocus sativus). Clinica Chimica Acta 2005; 352: 155-163
- Devasagayam TPA, Boloor KK, Mishra KP. Some new methods for free radical research. SFRR- India Bulletin 2003; 2(2): 20-28
- Da Silva MC, Paiva SR. Antioxidant activity and flavonoid content of Clusia fluminensis Planch. & Triana. Annals of the Brazilian Academy of Sciences. 2012; 84(3): 609-616
- Komiyama K, Funayama S, Anraku Y, Mita A, Takahashi Y, Omura S, Shimasaki H. Isolation of isoflavonoids possessing antioxidant activity from the fermentation broth of Streptomyces sp. Journal of Antibiotics 1989; 42(9): 1344-1349
- Chang HB, Kim J. Antioxidant properties of dihydroherbimycin A from a newly isolated Steptomyces sp. Biotechnology Letters 2007; 29(4): 599-603
- He F, Yang Y, Yang G, Yu L. Components and antioxidant activity of the polysaccharide from Streptomyces virginia H03. Z. Naturforsch C. 2008; 63(3-4): 181-188
- Kim K, Kim M, Jung J. Antitumor and antioxidant activity of protocatechualdehyde produced from Streptomyces lincolnensis M-20. Archives of Pharmacal Research 2008; 31(12): 1572-1577
- Thenmozhi M, Sindhura S, Kannabiran K. Characterization of antioxidant activity of Streptomyces species VITTK3 isolated from Puducherry Coast, India. Journal of Advanced Scientific Research 2010; 1(2): 46-52
- Diraviyam T, Radhakrishnan M, Balagurunathan R. Antioxidant activity of melanin pigment from Streptomyces species D5 isolated from Desert soil, Rajasthan, India. Drug Invention Today 2011; 3(3): 12-13
- Bondent V, Brand-Williams W, Bereset C. Kinetic and mechanism of antioxidant activity using the DPPH free radical methods. LWT 1997; 30: 609- 615
- Chung Y, Chien C, Teng K, Chou S. Antioxidative and mutagenic properties of Zanthoxylum ailanthoides Sieb & zucc. Food Chemistry 2006; 97: 418-425
- Kaviarasan S, Naik GH, Gangabhagirathi R, Anuradha CV, Priyadarshini KI. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chemistry 2007; 103: 31-37
- Hsu B, Coupar IM, Ng K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chemistry 2006; 98: 317-328










