Keywords
Blastocystis hominis, Immunocompromised, Leukemia, Trichrome
Introduction
Blastocystis hominis (B. hominis) is one of the most common parasites isolated from stool specimens in symptomatic and asymptomatic persons. It is transmitted by oro-fecal route. Such unicellular protozoan has three major forms; vacuolar, granular, and amoeboid. The vacuolated form (10-30 μm) was the most frequently detected in fecal specimens. It was considered as harmless yeast, but it is now getting acceptance as an agent of human intestinal disease especially under immunosuppressive conditions [1-3]. Clinical features of illness that have been attributed to B. hominis are variable ranging from mild diarrhoea to acute gastroenteritis [4,5].
B. hominis is cosmopolitan in distribution with omnipresence [6]. It is also the most frequently isolated parasite in epidemiological surveys [7]. Prevalence varies world widely and even within various communities of the same country. Developing countries, as usual, have higher prevalences of the parasite (30%-50%) than developed countries (1.5%-10%) this has been attributed to poor hygiene, exposure to animals, and consumption of contaminated food or water [8].
Infection appears to be common in immunocompromised patients. Incidence of B. hominis among them was reported between 12.2% and 23.3% [9]. It was reported in 14.1% of children with leukemia and in 5.7% of controls in a case control study to detect the intestinal parasitic infection in Tehran, Iran. Leukemia is the most prevalent malignancy in children below 15 years. It occurs due to malignant deformity of stem cells. A large number of leukemic children may become victims of infections that are considered one of the lethal causes in leukemia [10].
Aim of the Work
Aim of the work is to determine the frequency of B. hominis infection among immunocompetent and immunocompromised children and to correlate some sociodemographic, hygienic and symptomatic factors with B. hominis infection.
Materials and Methods
Study setting
The sample was collected from Alexandria University children’s Hospital.
Study design and sample
It is a case series study, 55 children having acute lymphocytic leukemia were enrolled in the study, and they were aged 2-14 years. Forty-six of immunocompetent children were chosen as matched by age and sex to represent the control group; they included the siblings of leukemic cases and others from different departments who had no immunodeficient disorders. Consent was taken from the mothers of the target children.
Data collection
An interviewing questionnaire was designed to collect data from the hospital’s reports and mothers of target children, it included the following data: a) Demographic data, such as residence, occupation of their fathers, housing conditions regarding crowding index, source of water supply etc. In addition, it included data about personal hygiene and habits such as hand washing, nail trimming etc. b) Hospital’s report included GIT manifestations as vomiting, colic, diarrhoea, and general manifestations as loss of weight, loss of appetite and jaundice.
Microscopical examination
It included examination of stool specimens. A portion of the specimen was used to prepare thin smears for staining by trichrome stain [11,12].
Statistical analysis [13]
Data were coded, tabulated, and analyzed using computer program SPSS version 15. Chi-square (X2) test was used for testing the association between categorical variables. In case of small frequency, [Fisher Exact test (FEt) in a 2 ×2 tables] was used instead. Odds ratio (OR) was used to determine the risk factors affecting parasitic infections. The associated 95% confidence interval was also calculated. OR was considered statistically significant if the confidence don’t include 1.00
Results
It was found that about 67.4% of controls had B. hominis infection and shown only in 54.5% of the immunocompromised ones (Table 1).
| |
Cases (n= 55) |
Control (n= 46) |
| No. |
% |
No. |
% |
| +ve |
30 |
54.5 |
31 |
67.4 % |
| -ve |
25 |
45.5 |
15 |
32.6 % |
| χ2 (p) |
1.728 (0.223) |
Table 1: Percentage of Blastocystis hominis infection detected among leukemic patients and control.
Infection was higher among control (71.4%) than immunocompromised individuals were (50%) in the age group <5 years.
It was found that both male and female of control group had higher percentage of infection (60.7% and 77.8% respectively) than immunocompromised children (57.9 and 47.1% in order).
As regard father occupation, it was observed that the percentage of infection was equaled among immunocompromised and controls in children whose fathers were professional. While statistically, insignificant higher percentages were observed among controls than leukemic cases in all other groups [FEp>0.05].
Regarding area of residence, it was found that either in rural or urban areas the percentage of infection were higher among controls (71.4% and 65.6% respectively) than leukemic cases (51.4% and 60.0% respectively) (Table 2 and Figure 1).
| Socio demographic characteristics |
Cases (n= 55) |
Control (n= 46) |
χ2 (p) |
| Total examined No. |
Total infected No. |
% |
Total examined No. |
Total infected No. |
% |
| Age in years |
| <5 |
14 |
7 |
12.7 |
7 |
5 |
10.9 |
2.608 (0.271) |
| 5 - <10 |
27 |
17 |
30.9 |
19 |
14 |
30.4 |
| 10 - <15 |
14 |
6 |
10.9 |
20 |
12 |
26.1 |
| Gender |
| Male |
38 |
22 |
40 |
28 |
17 |
36.95 |
2.262 (0.133) |
| Female |
17 |
8 |
14.5 |
18 |
14 |
30.4 |
| Father occupation |
| Professional |
30 |
20 |
36.4 |
21 |
14 |
30.4 |
MCp=
0.261 |
| Employee |
3 |
1 |
1.8 |
10 |
5 |
1.9 |
| Farmer |
15 |
5 |
9.1 |
9 |
7 |
15.2 |
| Not work or died |
7 |
4 |
7.3 |
6 |
5 |
1.9 |
| Are of residence |
| Rural |
35 |
18 |
32.7 |
14 |
10 |
21.7 |
4.725* (0.030) |
| Urban |
20 |
12 |
21.8 |
32 |
21 |
45.7 |
| χ2: Chi square test; FEp: p value for Fisher Exact test; MCp: p for Monte Carlo test |
Table 2: Distribution of Blastocystis hominis among examined children according to socio demographic characteristics.
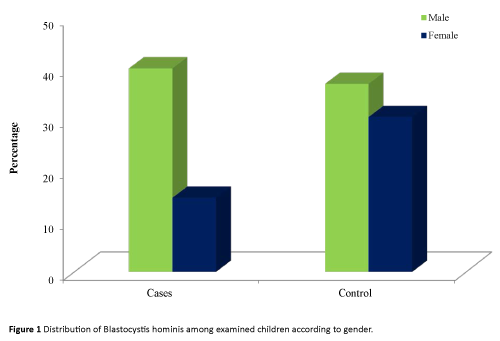
Figure 1: Distribution of Blastocystis hominis among examined children according to gender.
Studying the relevant symptoms in both immunocompromised and immunocompetent groups to declare the association between B. hominis and such symptoms; about 90% of the infected controls were asymptomatic versus 34.5% of the infected immunocompromised group. The difference was statistically significant (Table 3 and Figure 2).
| Environmental parameters |
Cases (n= 55) |
Control (n= 46) |
FEp |
| Total examined No. |
Total infected No. |
% |
Total examined No. |
Total infected No. |
% |
| Source of drinking water |
| Private tap water |
45 |
25 |
45.5 |
41 |
27 |
58.7 |
0.731 |
| Public tap water |
10 |
5 |
9.1 |
5 |
4 |
8.7 |
| Storage of water |
| None |
45 |
25 |
45.5 |
40 |
26 |
56.5 |
1.000 |
| Plastic container |
3 |
1 |
1.8 |
1 |
1 |
2.2 |
| Zir |
7 |
4 |
7.3 |
5 |
4 |
8.7 |
| Latrine |
| Private |
50 |
28 |
50.9 |
45 |
30 |
65.2 |
0.612 |
| Shared |
5 |
2 |
3.6 |
1 |
1 |
2.2 |
| Presence of animals in the house |
| Present |
13 |
6 |
10.9 |
7 |
5 |
10.9 |
0.749 |
| Not |
42 |
24 |
43.6 |
39 |
26 |
56.5 |
| Crowding index CI |
| Low |
34 |
17 |
30.9 |
36 |
24 |
52.2 |
0.082 |
| Moderate |
18 |
12 |
21.8 |
7 |
5 |
10.9 |
| High |
3 |
1 |
1.8 |
3 |
2 |
4.3 |
| Low CI: <3 persons/room; Moderate CI: 3<6 persons/room; High CI: ≥6 persons/room; FEp: p value for Fisher Exact test; *: Statistically significant at p ≤ 0.05. |
Table 3: Distribution of Blastocystis hominis among immuno compromised and immuno competent according to environmental parameters.
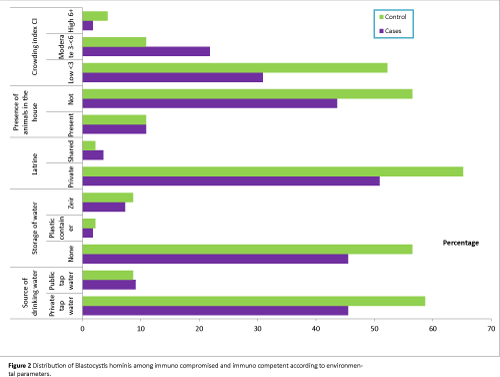
Figure 2: Distribution of Blastocystis hominis among immuno compromised and immuno competent according to environmental parameters.
All the studied socioeconomic parameters were associated with higher percentages of infection among controls than cases of leukemia. Use of private tap water was associated with B. hominis infection in 55.6% of cases and 65.9% of controls. Same pattern was observed among those who used public tap water in whom percentage of infection were 50% among leukemic cases versus 80% among controls.
As regard storage of water, also higher percentages of infection were noticed among controls than cases whether they did not store water or stored water in plastic container or zir.
Regarding use of latrine, it was observed that either they use private or shared latrines, statistically insignificant higher percentages of infection were recorded among controls (66.7% and 100% respectively) than leukemic cases (56% and 40% respectively).
Percentages of infection were higher among controls than immunocompromised whether animals were present or not.
All groups of crowding levels showed higher percentage of infection among controls than immunocompromised cases. At the same time, the highest percentage of infection was observed among leukemic cases (66.7%) and controls (71.41%) of moderate crowding index level than low or high levels. However, no statistical significant differences were detected (Figure 1).
There is an astonishing peculiar phenomenon shown in the association between personal hygiene and distribution of infection among examined immunocompetent children. As the percentage of infection increased with almost all parameters where hygienic manner was applied. Whereas statistically insignificant lower percentage of infection was only seen among those of immunocompetent group who did nail trimming (66.7%) than those who did not (68.0%)
On the other hand higher percentages of infection among immunocompromised children were noticed when they do not follow hygienic maneuvers, eat food outdoors, play in the street and do not trim their nails. The difference in the two former conditions was statistically significant. (OR=5.45, 12.46). The reverse was obtained when they wash their hands before eating and after defecation and when they wash vegetables (Table 4).
| Personal habits and hygiene |
Cases (n= 55) |
Control (n= 46) |
χ2 (p) |
| Total examined No. |
Total infected No. |
% |
Total examined No. |
Total infected No. |
% |
| Eating food outdoors |
| No |
29 |
13 |
23.6 |
35 |
25 |
45.5 |
9.036* (0.003) |
| Yes |
26 |
17 |
30.9 |
11 |
6 |
10.9 |
| Washing hands before eating |
| No |
45 |
24 |
43.6 |
28 |
17 |
30.9 |
4.380* (0.036) |
| Yes |
10 |
6 |
10.9 |
18 |
14 |
25.5 |
| Washing hands after defecation |
| No |
44 |
23 |
41.8 |
26 |
16 |
29.1 |
4.150* (0.042) |
| Yes |
11 |
7 |
12.7 |
20 |
15 |
27.3 |
| Washing vegetables before eating |
| No |
27 |
14 |
25.5 |
17 |
10 |
18.2 |
1.326 (0.249) |
| Yes |
28 |
16 |
29.1 |
29 |
21 |
38.2 |
| Play in the street |
| No |
10 |
3 |
5.5 |
25 |
18 |
32.7 |
FEp < 0.001* |
| Yes |
45 |
27 |
49.1 |
21 |
13 |
23.6 |
| Nail trimming |
| No |
22 |
14 |
25.5 |
25 |
17 |
30.9 |
0.407 (0.523) |
| Yes |
33 |
16 |
29.1 |
21 |
14 |
25.5 |
| χ2: Chi square test; FEp: p value for Fisher Exact test; *: Statistically significant at p ≤ 0.05. |
Table 4: Distribution of Blastocystis hominis infection among immuno compromised and immune competent in relation to personal habits and hygiene.
Table 5 shows that there is no significant association between complaints and infection with B. hominis.
| |
Blastocystis hominis |
Total |
| - ve |
+ ve |
| No |
% |
No |
% |
No |
% |
| Pain |
| No |
31 |
72.1 |
43 |
74.1 |
74 |
73.3 |
| Yes |
12 |
27.9 |
15 |
25.9 |
27 |
26.7 |
| χ2 (p) |
0.053 (0.818) |
|
|
| Fever |
| No |
34 |
79.1 |
49 |
84.5 |
83 |
82.2 |
| Yes |
9 |
20.9 |
9 |
15.5 |
18 |
17.8 |
| χ2 (p) |
0.494 (0.482) |
|
|
| Tirdness weakness |
| No |
43 |
100.0 |
54 |
93.1 |
97 |
96.0 |
| Yes |
0 |
0.0 |
4 |
6.9 |
4 |
4.0 |
| FEp |
0.134 |
|
|
| Difficulty in breathing |
| No |
41 |
95.3 |
55 |
94.8 |
96 |
95.0 |
| Yes |
2 |
4.7 |
3 |
5.2 |
5 |
5.0 |
| FEp |
1.000 |
|
|
| Difficulty in swallowing |
| No |
43 |
100.0 |
55 |
94.8 |
98 |
97.0 |
| Yes |
0 |
0.0 |
3 |
5.2 |
3 |
3.0 |
| FEp |
0.259 |
|
|
| Loss of appetite |
| No |
39 |
90.7 |
47 |
81.0 |
86 |
85.1 |
| Yes |
4 |
9.3 |
11 |
19.0 |
15 |
14.9 |
| FEp |
0.259 |
|
|
| Loss of weight |
| No |
40 |
93.0 |
48 |
82.8 |
88 |
87.1 |
| Yes |
3 |
7.0 |
10 |
17.2 |
13 |
12.9 |
| FEp |
0.147 |
|
|
| Jaundice |
| No |
40 |
93.0 |
56 |
96.6 |
96 |
95.0 |
| Yes |
3 |
7.0 |
2 |
3.4 |
5 |
5.0 |
| FEp |
0.648 |
|
|
| χ2: Chi square test; FEp: p value for Fisher Exact test. |
Table 5: Comparison between negative and positive Blastocystis hominis according to history of complaints.
Discussion
B. hominis was the most common parasite isolated from stool samples [14,15]. Higher prevalence among immunocomporomised is questionable. According to the literature, developing countries have higher prevalence of the parasite (30%-50%) than developed countries (1.5%-10%) [16-18]. In the present study 67% were recorded among controls and 55% among leukemic cases. The lower percentages among immunocompromised could be attributed to pharmacologic effect of cytotoxic drugs upon the parasite [10].
Leukemic children under treatment may be less active, are losing appetite, and have more care from their mothers so they are less exposed to infection than normally active ones.
Similar information was documented among immunocompetent (71.8% B. hominis infection) by a laboratory in Ethiopia [19]. So that being immunocompromised is not a cause of parasitic infection.
In 2007, Ozcakir et al. [1] also reported that B. hominis incidence in the patient group was lower (11.2%) than healthy volunteers (13%), using trichrome staining technique.
Our study showed that the highest prevalence of B. hominis infection among both immunocompromised and immunocompetent children aged 5<10 years old than younger and older groups.
These results were in agreement with Nascimento and Moitinho [20], against that reported by Yaicharoen et al. [21] whose study showed no definite pattern of the relationships between age and infection rate, attributed it to multifactorial etiology.
As regard father occupation the highest prevalence was among immunocompetent children, only whose fathers were farmers. This could be explained by being residents in hospital for leukemia therapy. The same happened regarding area of residence that the highest prevalence was in immunocompetent children of rural area.
Statistical analysis showed no significant difference between male and female. However, Hegazy et al. [22] reported that the prevalence of B. hominis in males was higher than females (60.5% versus 39.5%)
B. hominis a parasite with doubtful pathogenicity in an immunocompetent host. Opportunistic subtype could cause symptoms in immunocompromised cases.
In the present study, about 90% of infected immunocompetent children were asymptomatic. This could be explained by recent data that demonstrated the pathogenicity of a parasite is subtype-dependent. Such subtypes are different pathogenic and non-pathogenic strains [23].
Other authors suggested co-occurrence of B. hominis infection with other pathogenic parasites might confuse its pathogenic potential [24].
The association of clinical symptoms and B. hominis even in immunocompromised children could be ignored as some authors documented that all patients improved without receiving any specific therapy [25].
On the contrary, some authors recommended proper diagnosis and management of the disease with emphasis on its pathogenic potential [19].
In this research we found patients with immunocompromised system and ready for infections had lower infection level than immunocompetent ones.
Although there was positive association between B. hominis infection and bad environmental parameters in immunocompetent children, immunocompromised children had negative association.
The same trend was seen, regarding hygienic parameters in both studied groups, where good hygienic manners were positively associated with B. hominis infection.
This is in agreement with others who found high prevalence among preschool children residing in an urban sitting and having good access to sanitary services [3,16].
In addition, high infection role of B. hominis is directly proportional to hygienic and sanitary conditions of the studied population.
Kassem et al. [26] also stated that high prevalence of B. hominis infection was in moderate socioeconomic status and no significant effect of crowding index.
However playing in the street and eating food outdoors were risk factors for B. hominis infection among immunocompromised children where 95% CI=(2.72-65.33) and (1.52-20.39) respectively.
This is in accordance with others attributed high prevalence of B. hominis infection to living in poor hygienic conditioned environment [27].
Considering associated manifestations, some authors didn’t consider its presence in stool of immunocompromised or immunocompetent to be any more significant than that of Entamoeba coli or E. dispar [28]. Similar information was reported by the present study.
Conclusion
The data suggest that the isolation of B. hominis does not necessitate treatment even in immunocompromised patients since its pathogenicity is debatable. Unless there is more convincing evidence of pathogenicity efforts should be saved for remedying serious illness of vulnerable groups. Further studies on molecular basis are recommended.
Acknowledgement
We would like to thank Dr. Faika Ibrahim M. Hassanein, Master of Public Health (Parasitology and Medical Entomology) for helping us during performance of practical studies.
9383
References
- Ozçakir O, Güreser S, Ergüven S, Yilmaz YA, Topaloglu R, et al. (2007) Characteristics of Blastocystis hominis infection in a Turkish university hospital. Turkiye Parazitol Derg 31: 277-282.
- El-Shazly AM, Abdel-Magied AA, El-Beshbishi SN, El-Nahas HA, Fouad MA, et al. (2005) Blastocystis hominis among symptomatic and asymptomatic individuals in Talkha Center, Dakahlia Governorate, Egypt. J Egypt Soc Parasitol 35: 653-666.
- Al FD, Hökelek M (2007) Is Blastocystis hominis an opportunist agent? Turkiye Parazitol Derg 31: 28-36.
- Kaya S, Cetin ES, AridoÄŸan BC, Arikan S, Demirci M (2007) Pathogenicity of Blastocystis hominis, a clinical reevaluation. Turkiye Parazitol Derg 31: 184-187.
- Andiran N, Acikgoz ZC, Turkay S, Andiran F (2006) Blastocystis hominis--an emerging and imitating cause of acute abdomen in children. J Pediatr Surg 41: 1489-1491.
- Tan KS (2004) Blastocystis in humans and animals: new insights using modern methodologies. Vet Parasitol 126: 121-144.
- Baldo ET, Belizario VY, De Leon WU, Kong HH, Chung DI (2004) Infection status of intestinal parasites in children living in residential institutions in Metro Manila, the Philippines. Korean J Parasitol 42: 67-70.
- Wong KH, Ng GC, Lin RT, Yoshikawa H, Taylor MB, et al. (2008) Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol Res 102: 663-670.
- Mohandas SR, Sud A, Malla N (2002) Prevelance of intestinal parasitic pathogens in HIV- seropositive cases in Northern India. Jpn J Infect Dis 55: 83-84.
- Gharavi MJ, Ashraf F, Vosough P, Rokni MB (2003) Survey of Intestinal Parasitic Infection in Leukemic Children and Evaluation of their Serum Immunoglobulins. Iranian J Publ Health 32: 19-21.
- Garcia LS (2001) Diagnostic Medical Parasitology (4thedn). ASM press. Washington DC 723.
- Dogruman-Al F, Turk S, Adiyaman-Korkmaz G, Hananel A, Levi L, et al. (2015) A novel ELISA test for laboratory diagnosis of Blastocystis spp. in human stool specimens. Parasitol Res 114: 495-500.
- Jekwl JF, Katz DL, El more JG (2001) Epidemiology, Biostatistics and Preventive medicine (2ndedn) Philadelphia, London, New York, St Louis, Sydney, Toronto: WB Saunders Co.
- Danniel WW (1995) Biostatistics. A foundation for Analysis in the Health Science (6thedn) New York, Chichester, Brishane, Toronto, Singapore: John Wiley and Sons, Inc.
- Tanizaki A, Yoshikawa H, Iwatani S, Kimata I (2005) Infectivity of Blastocystis isolates from chickens, quails and geese in chickens. Parasitol Res 96: 57-61.
- Hassanein FI (2012) Association of inflammatory bowel diseases and irritable bowel syndrome with some intestinal parasitic infections (Doctor thesis). Alexandria: High Institute of Public Health, Alexandria University.
- Londoño AL, Mejía S, Gómez-Marín JE (2009) (Prevalence and risk factors associated with intestinal parasitism in preschool children from the urban area of Calarcá, Colombia). Rev Salud Publica (Bogota) 11: 72-81.
- Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA (2008) Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One 3: e3680.
- Endeshaw T, Tadesse G, Petros B (2007) significance of Blastocystis hominis in patients referred for bacteriological stool culture at EHNRI. Ethiop. J Health Dev 21: 61-66.
- Nascimento SA, Moitinho ML (2005) B-hominis and other intestinal parasites in a community of Pitango city, parana state, Brazil. Revista do institute de Medicine Tropical de Säo Paulo 47: 213-217.
- Yaicharoen R, Ngrenngarmlert W, Wongjindanon N, Sripochang S, Kiatfuengfoo R (2006) Infection of Blastocystis hominis in primary schoolchildren from NakhonPathom province, Thailand. Trop Biomed 23: 117-122.
- Hegazy MM, Maklouf LM, El Hamshary EM, Dawoud HA, Eida AM (2008) Protein profile and morphometry of cultured human Blastocystis hominis from children with gastroenteritis and healthy ones. J Egypt Soc Parasitol 38: 453-464.
- Hussein EM, Hussein AM, Aida MM, Atwa MM (2008) Pathophysiological variability of different geno-types of human B. hominis Egyptian isolates in experimentally infected rats. Parasitol Res 102: 853-860.
- Bayomy AM, Mohamed AA, Ghannam MA, Shahat SA, Saadawy AK (2010) Opportunistic parasitic infections among immunocompromised Egyptian patients. J Egypt Soc Parasitol 40: 797-808.
- Kuo HY, Chiang DH, Wang CC, Chen TL, Fung CP, et al. (2008) Clinical significance of B. hominis: Experience from a medical center in Northern Taiwan. J Microbial immunol Infect 41: 222-226.
- Kasssem HH, Zaed HA, Sadaga GA (2007) Intestinal parasitic infection among children and neonatus admitted to Ibn-Sina Hospital, Sirt, Libya. J Egypt Soc Parasitol 37: 371-380.
- Borges JD, Alarcon RS, Neto VA, Cakiyo E (2009) Intestinal parasitosis in Indians of the Mapura community (Oriximina, state of Para, Brazil): High prevalence of B. hominis and finding of Cryptosporidium sp. And Cyclosporacayetanensis. Rev Soc Bras Med Trop 42: 348-350.
- Markell EK, Voge M (2006) Parasitic infections in immunocompromised hosts. Medical Parasitology. Elsevier. Inc 353.








