Introduction
|
| |
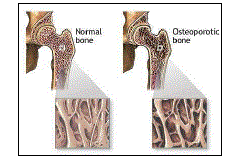
| Osteoporosis is a disease of bones that leads to an increased risk of fracture. Osteoporosis literally means 'porous bones'. The two Greek words which make up the term osteoporosis are "osteon" which means bone and "poros" which means pore 1. |
| |
| “Osteo” means bone. Osteoporosis is a condition that is characterized by a decrease in the density of bone. It is often referred to as the “silent disease” because bone loss occurs without symptoms. |
| |
| The term osteoporosis was coined by Johann Lobstein at about the same time, but the disorder he described was probably osteogenesis imperfecta5 In osteoporosis the bone mineral density (BMD) is reduced and the bone’s microarchitecture is deteriorated, and the amount and variety of proteins in bone is altered. Osteoporosis is defined by the World Health Organization (WHO) as a bone mineral density that is 2.5 standard deviations or more below the mean peak bone mass (average of young, healthy adults) as measured by DXA; the term "established osteoporosis" includes the presence of a fragility fracture 2. |
| |
| Recently published data have clearly demonstrated widespread vitamin D deficiency across India, at all ages and in both sexes, particularly in the urban areas. Poor sunlight exposure, skin pigmentation and a vitamin D-deficient diet are some obvious causes for this finding. Indians have low BMD as compared to the western Caucasians. |
| |
| The disease may be classified as either primary type 1 or type 2 and secondary osteoporosis.1 Osteoporosis is most common in women after menopause, and is referred to as primary type 1 or post menopausal osteoporosis. Primary type 2 osteoporosis or senile osteoporosis occurs at age 75 years and older and is seen in both females and males in a 2:1 ratio. The onset of secondary osteoporosis is at any age, and affects both men and women equally. This type of osteoporosis is a result of chronic or prolonged use of certain medications and the presence of predisposing medical problems or disease states. Therefore, osteoporosis may also develop in men, and may occur in anyone in the presence of particular hormonal disorders and other chronic diseases or as a result of medications, specifically glucocorticoids, when the disease is called steroid- or glucocorticoid-induced osteoporosis (SIOP or GIOP). Given its influence in the risk of fragility fracture, osteoporosis may significantly affect life expectancy and quality of life. During puberty and adolescence, the skeleton takes up calcium avidly and builds up its reserves. This uptake of calcium into the bone is largely dependent on calcium and vitamin D nutrition, as well as exercise. Peak bone mass is usually achieved by the age of 30. From the mid-thirties there is a gradual, progressive bone loss, which continues throughout life and is accelerated at the menopause in women. The fracture prevention strategy therefore consists of increasing peak bone mass in the growing years and reducing subsequent bone loss throughout life. Thus, the importance of achieving and maintaining good bone health cannot be over emphasized.3 |
| |
| The pathogenesis of osteoporosis is complex. In childhood and adolescent period bone formation exceeds resorption, resulting in continued skeletal growth and denser, longer and heavier bones. This process slows down in adulthood, and peak bone mass is attained at about 30 yr of age. After this, resorption begins to exceed formation. Normal bone loss averages 0.7 per cent per year. It gets accelerated at the time of menopause to 2-5 per cent per year, which may continue for up to 10 years. Since cancellous bone is much more metabolically active than cortical bone, in periods of accelerated bone loss cancellous bone loss is 3-fold greater. Osteoporotic fractures, therefore, commonly occur in vertebrae. Peak bone mass is primarily determined by genes but may be modified to a considerable extent by certain factors like physical activity, calcium, vitamin D nutrition, smoking, alcohol, concurrent illnesses, and medications (glucocorticoids, Antiepileptics) 4. The level of peak bone mass achieved at puberty is a major determinant of bone mass in later life and hence an important factor in the ultimate development of osteoporosis. |
| |
|
Signs and Symptoms
|
| |
| Osteoporosis may be caused due to some of the following reason; |
| |
| · Endocrine disorders such as diabetes |
| |
| · Drug side effects |
| |
| · Long term use of corticosteroid |
| |
| · Rheumatoid arthritis |
| |
| · Prolonged bed rest |
| |
| The symptoms associated with osteoporosis are as follow; |
| |
| · Low back pain |
| |
| · Bone pain in the hip, arm or wrist |
| |
| · Loss of height or a stooped posture |
| |
| · Neck pain |
| |
| · Fractures of the hip, spine, back or wrist, sometimes even without falling |
| |
|
Low-Energy Fractures
|
| |
| Breaking a bone with minimal force, is when we need to worry about osteoporosis . |
| |
|
Unexplained Bone or Joint Pain
|
| |
| There are many causes of bone and joint pain, but osteoporosis may contribute to these symptoms. When the bones lack sufficient strength to hold the weight of your body, injury can occur. Unexplained bone or joint pain may raise the consideration of a bone health problem. |
| |
|
Height Loss or Stooping
|
| |
| Compression fractures of the spine may go undetected or be attributed to a back strain type of injury. When multiple vertebrae are injured, people may lose height or develop a curvature to their spine. The typical appearance of an individual with compression fractures is a short stature with a humped back. |
| |
| By the age of 20, an average woman acquires 98% of her skeletal mass. This process of bone acquisition slows as one gets older. Between the age of 35-40, women begins to lose their bone mass; this loss remains reasonably under control until the age of 50 and then declines progressively. This loss, if severe, can lead to osteoporosis. |
| |
|
Risk Factors & Causes
|
| |
| The following are the factors that will increase the risk of developing osteoporosis; |
| |
| · Sex: Female gender |
| |
| · Age: Personal history of fracture as an adult |
| |
| · Race: Caucasian or Asian race |
| |
| · Family history: Family history of osteoporosis (for example, having a mother with an osteoporotic hip fracture doubles your risk of hip fracture) |
| |
| · Frame size: Thin and small body frame |
| |
| · Cigarette smoking |
| |
| · Excessive alcohol consumption |
| |
| · Lack of exercise |
| |
| · Diet low in calcium |
| |
| · Poor nutrition and poor general health |
| |
| · Lifetime exposure to estrogen: 1. Early menopause; 2. Infrequent menstruation periods |
| |
| · Eating disorders: Anorexia nervosa or bulimia (lower bone density in lower backs and hips) |
| |
| · Medications: Long term use of corticosteroid, antiseizure medications, diuretics, aluminum containing antacids |
| |
|
Bone Formation
|
| |
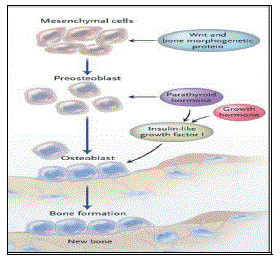
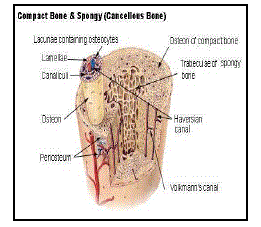
| Bone tissue is a mineralized connective tissue. It is formed by cells, called osteoblasts, that deposit a matrix of Type-I collagen and also release calcium, magnesium, and phosphate ions that ultimately combine chemically within the collagenous matrix into the crystalline mineral hydroxyapatite. The combination of hard mineral and flexible collagen makes bone harder and stronger than cartilage without being brittle. The primary anatomical and functional unit of mammalian compact bone consists of a repeating structure called Haversian system, or osteon. Each osteon has concentric layers of mineralized matrix, called concentric lamellae, which are deposited around a central canal, also known as the Haversian canal, containing blood vessels and nerves that service the bone. |
| |
|
Types of bone
|
| |
| Osseous tissue, or Calcium tissue, is the major structural and supportive connective tissue of the body. Osseous tissue forms the rigid part of the bone organs that make up the skeletal system. There are two types of osseous tissue; |
| |
| · Compact : Compact bone forms the extremely hard exterior while |
| |
| · Spongy : Spongy bone fills the hollow interior. |
| |
| The tissues are biologically identical; the difference lies in how the microstructure is arranged. |
| |
|
Functions
|
| |
| · Osseous tissue performs numerous |
| |
| · Support for muscles, organs, and soft tissues. |
| |
| · Leverage and movement. |
| |
| · Protection of vital organs. e.g. the heart (Note: some vital organs may not be protected by bones. e.g. the intestines.) |
| |
| · Calcium phosphate storage. Indirectly: |
| |
| Hemotopoiesis - formation of blood cells (more correctly this is performed by the bone marrow interspersed within the spongy interior). |
| |
|
Theory
|
| |
| In 1940, the American physician and endocrinologist Fuller Albright described postmenopausal osteoporosis and proposed that it was the consequence of impaired bone formation due to estrogen deficiency 6. Subsequently, the concept that there are 2 forms of osteoporosis, one related to estrogen deficiency at the menopause and the other to calcium deficiency and aging of the skeleton was proposed 7 |
| |
| There is a rapidly expanding amount of information, based on laboratory studies, that indicates that osteoporosis is likely to be caused by complex interactions among local and systemic regulators of bone cell function. The heterogeneity of osteoporosis may be due not only to differences in the production of systemic and local regulators, but also to changes in receptors, signal transduction mechanisms, nuclear transcription factors, and enzymes that produce or inactivate local regulators8. Osteoporosis study indicated an association among bone mass, fragility, and polymorphisms in the vitamin D receptor (VDR) gene, more than 30 candidate genes have been reported that might influence skeletal mass and fragility (9,10). |
| |
| Since VDR gene may be a determinant of bone mass, differences in VDR gene polymorphism in different races could account for differences in bone mass. Polymorphism of the alleles of the vitamin D receptor gene may account for the major part of the heritable component of bone density in women, possibly mediated in part by impaired calcium absorption from the bowel but this association has not been found in group of men.4 |
| |
| gene may account for the major part of the heritable component of bone density in women, possibly mediated in part by impaired calcium absorption from the bowel but this association has not been found in group of men.4 |
| |
| Fractures - The symptoms of a vertebral collapse are sudden back pain, often with radiculopathic pain (shooting pain due to nerve root compression) and rarely with spinal cord compression or cauda equina syndrome. Multiple vertebral fractures lead to a stooped posture, loss of height, and chronic pain with resultant reduction in mobility28 |
| |
|
Pathogenesis
|
| |
| The underlying mechanism in all cases of osteoporosis is an imbalance between bone resorption and bone formation. In normal bone, there is constant matrix remodeling of bone; up to 10% of all bone mass may be undergoing remodeling at any point in time. The process takes place in bone multicellular units (BMUs) as first described by Frost in 196329 Bone is resorbed by osteoclast cells (which derive from the bone marrow), after which new bone is deposited by osteoblast cells.30 |
| |
| Skeletal fragility can result from (a) failure to produce a skeleton of optimal mass and strength during growth; (b) excessive bone resorption resulting in decreased bone mass and micro architectural deterioration of the skeleton; and (c) an inadequate formation response to increased resorption during bone remodeling. In addition, the incidence of fragility fractures, particularly of the hip and wrist, is further determined by the frequency and direction of falls. Peak bone mass is primarily determined by genes but may be modified to a considerable extent by certain factors like physical activity, calcium, vitamin D nutrition, smoking, alcohol, concurrent illnesses, and medications (glucocorticoids, antiepileptics)11 |
| |
| Diagnosis: The diagnosis of osteoporosis can be made using conventional radiography and by measuring the bone mineral density (BMD).31 The Bone mineral density testing (specifically a densitometry or DEXA scan) measures how much bone one has. |
| |
| There are many options available to diagnose, treat and follow the progress of osteopenia and osteoporosis32-34 There are image-based exams that allow density quantification of trabecular and cortical bone in a restricted area or in the entire body; each one with a precise indication35-37. |
| |
| Health professionals have been searching for substances that can help increase the density of osteoporotic bones. That can be achieved either by diminishing bone resorption or improving bone formation. Bisphosphonates are being used widely in diseases that present increase in bone resorption, such as senile or post-menopause osteoporosis. Among bisphosphonates, risedronate shows a higher anti-resorptive effect38-40. Risedronate acts as an osteoclast inhibitor. |
| |
| When a fracture or lesion occurs, the body mobilizes cells such as osteoclasts and osteoblasts to repair the injured bone. The osteoclasts absorb the necrotic bone and remodel the new bone created by osteoblasts that proliferate intensively and produce the new matrix. These cells work simultaneously forming an immature bone callus, which shows progressive remodeling until complete replacement by mature bone33 |
| |
| In osteoporotic bones, the osseous turnover does not occur in an adequate way because in the remodeling process bone formation is diminished and resorption is increased41 |
| |
| Conventional Radiography: Conventional radiography is useful, both by itself and in conjunction with CT or MRI, for detecting complications of osteopenia (reduced bone mass; pre-osteoporosis), such as fractures. |
| |
|
Dual energy X-ray absorptiometry
|
| |
| Dual energy X-ray absorptiometry (DXA, formerly DEXA) is considered the gold standard for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the bone mineral density is less than or equal to 2.5 standard deviations below that of a young adult reference population. This is translated as a Tscore. 43 |
| |
| Clinical Decision Rule: A number of clinical decision rules have been created to predict the risk of osteoporotic fractures. The Q Fracture score was developed in 2009 and is based on age, BMI, smoking status, alcohol use, rheumatoid arthritis, cardiovascular disease, type 2 diabetes, asthma, use of tricyclic antidepressants or corticosteroids, liver disease, and a history of falls in men. In women hormone replacement therapy, parental history of osteoporosis, gastrointestinal malabsorption, and menopausal symptoms are also taken into account |
| |
| Treatment: There are several alternatives of medication to treat osteoporosis, depending on gender, though lifestyle changes are also very frequently an aspect of treatment. |
| |
|
Medication:
|
| |
| Bisphosphonates are the main pharmacological measures for treatment. However, newer drugs such as teriparatide and strontium ranelate are also used. |
| |
| Bisphosphonates: In confirmed osteoporosis, bisphosphonate drugs are the first-line treatment in women. The most often prescribed bisphosphonates are presently sodium alendronate (Fosamax) 10 mg a day or 70 mg once a week, risedronate (Actonel) 5 mg a day or 35 mg once a week and or ibandronate (Boniva) once a month. The currently FDA-approved bisphosphonates alendronate, risedronate, and ibandronate — have been shown to reduce the incidence of fractures, including both vertebral and nonvertebral fractures, particularly hip fractures56, 57 These agents bind to the bone surface and then are Bisphosphonates: In confirmed osteoporosis, bisphosphonate drugs are the first-line treatment in women. The most often prescribed bisphosphonates are presently sodium alendronate (Fosamax) 10 mg a day or 70 mg once a week, risedronate (Actonel) 5 mg a day or 35 mg once a week and or ibandronate (Boniva) once a month. The currently FDA-approved bisphosphonates alendronate, risedronate, and ibandronate — have been shown to reduce the incidence of fractures, including both vertebral and nonvertebral fractures, particularly hip fractures56, 57 These agents bind to the bone surface and then are |
| |
| Mode of Action: Bisphosphonates are antiresorptive medicines, which means they slow or stop the natural process that dissolves bone tissue, resulting in maintained or increased bone density and strength.59 This may prevent the development of osteoporosis. If osteoporosis already has developed, slowing the rate of bone thinning reduces the risk of broken bones. Certain studies show that bisphosphonates increase bone thickness and lower the risk of fractures. If you take alendronate or risedronate, you may not be as likely to break a bone60, 61 |
| |
| Bisphosphonates, should be taken along with calcium and vitamin D supplements. But calcium supplements may interfere with the body's ability to absorb bisphosphonates, so they may be taken at different times in a day. |
| |
| Bisphosphonates are sometimes taken in combination with hormone replacement therapy (HRT) by women who are not getting increased bone thickness from a bisphosphonate alone. Studies show that this combination can increase bone thickness a little more than taking either a bisphosphonate or estrogen alone. But combining medicines also leads to increased costs and increased risk of side effects.62 |
| |
| Calcitonin (Calcimar, Miacalcin): This medication, a hormone made from the thyroid gland, is given usually as a nasal spray or as an injection under the skin. It has been FDA approved for the management of postmenopausal osteoporosis and helps prevent vertebral (spine) fractures. It also is helpful in controlling pain after an osteoporotic vertebral fracture.52 calcitonin deficiency was not found in osteoporotic patients, and calcitonin therapy has been less effective than other antiresorptive agents, possibly because osteoclasts can escape calcitonin inhibition56, 57 |
| |
| Calcitonin can be taken in a nasal spray, as a shot into the muscle (intramuscular, or IM), or as a shot into the fat tissue (subcutaneous). |
| |
| Mode of Action: Calcitonin is a naturally occurring hormone. It helps regulate calcium levels in the body and is involved in the process of bone building. When given by shot or nasal spray, it slows the rate of bone thinning. It also relieves pain that occurs when the bones in the spine (vertebrae) break and collapse on top of each other (spinal compression fracture). |
| |
| It is given to women who are more than 5 years beyond menopause and who do not tolerate bisphosphonate medications.63 |
| |
| Calcitonin slows thinning of bone in the spine, hip (pelvis), and ends of the long bones (trabecular bone). But it does not appear to be as effective as other medicines, such as raloxifene or alendronate, at building bone mass and reducing the risk of fractures.64 Calcitonin helps relieve pain from broken bones caused by osteoporosis |
| |
| Calcitonin slows thinning of bone in the spine, hip (pelvis), and ends of the long bones (trabecular bone). But it does not appear to be as effective as other medicines, such as raloxifene or alendronate, at building bone mass and reducing the risk of fractures.64 Calcitonin helps relieve pain from broken bones caused by osteoporosis well tolerated, with an ADE profile similar to that of placebo, except for the incidence of dizziness and leg cramps. Teriparatide appears to have no significant drug–drug interactions. |
| |
| Teriparatide has a place in therapy as an alternative treatment for osteoporosis, although no current studies have demonstrated its safety or efficacy after two years of use. |
| |
| Strontium ranelate: Oral strontium ranelate is an alternative oral treatment, belonging to a class of drugs called "dual action bone agents" (DABAs) by its manufacturer. It has proven efficacy, especially in the prevention of vertebral fracture43 Strontium ranelate is taken as a 2 g oral suspension daily, and is licensed for the treatment of osteoporosis to prevent vertebral and hip fracture. Strontium ranelate has side effect benefits over the bisphosphonates, as it does not cause any form of upper GI side effect, which is the most common cause for medication withdrawal in osteoporosis. Strontium, no matter what the form, must be water-soluble and ionized in the stomach acid. Stontium is then protein-bound for transport from the intestinal tract into the blood stream. Unlike drugs like sodium alendronate (Fosamax), strontium doesn't inhibit bone recycling and, in fact, may produce stronger bones. Strontium must not be taken with food or calcium-containing preparations as calcium competes with strontium during uptake. However, it's essential that calcium, magnesium, and vitamin D in theraputic amounts must be taken daily, but not at the same time as strontium. Strontium should be taken on an empty stomach at night. |
| |
| Hormone Replacement: Estrogen replacement therapy remains a good treatment for prevention of osteoporosis. |
| |
| Strontium ranelate is a drug that decreases the chance of fractures by slowing the loss of bone and possibly by building new bone. It is a new drug and therefore its benefits and harms need to be known66 . |
| |
| Selective estrogen receptor modulator (SERM): SERMs are a class of medications that act on the estrogen receptors throughout the body in a selective manner. Normally, bone mineral density (BMD) is tightly regulated by a balance between osteoblast and osteoclast activity in the trabecular bone. Estrogen has a major role in regulation of the bone formation-resorption equilibrium, as it stimulates osteoblast activity. Some SERMs such as raloxifene (Evista) act on the bone by slowing bone resorption by the osteoclasts44 In addition to treating bone loss, Raloxifene has a short-term impact on heart health. It has been shown to reduce levels of "bad" (or LDL) cholesterol and reduce levels of total cholesterol. |
| |
| Others, such as Femarelle (DT56a), achieve a significant effect by stimulating osteoblast activity thus inducing new bone formation45, similarly to the estrogenic effect. Both have been proved as effective in clinical trails46-48 |
| |
| Estrogen or Hormone Replacement Therapy: Estrogen therapy alone or in combination with another hormone, progestin, has been shown to decrease the risk of osteoporosis and osteoporotic fractures in women. However, the combination of estrogen with a progestin has been shown to increase the risk for breast cancer, strokes, heart attacks and blood clots. Estrogens alone may increase the risk of strokes. |
| |
| ERT is available in a variety of forms, such as oral tablets or topical patches applied to the skin, and can be made from a mixture of different naturally occurring estrogens or from a single type of estrogen. ERT only replaces the estrogen that stops being produced by the body during menopause. Estrogen therapy taken alone (ERT or unopposed estrogen) can increase a woman’s risk of developing cancer in the uterus (cancer of the uterine lining, called endometrial cancer). For women who have not had a hysterectomy, an additional hormone called progesterone, or a synthetic version called progestin ERT is available in a variety of forms, such as oral tablets or topical patches applied to the skin, and can be made from a mixture of different naturally occurring estrogens or from a single type of estrogen. ERT only replaces the estrogen that stops being produced by the body during menopause. Estrogen therapy taken alone (ERT or unopposed estrogen) can increase a woman’s risk of developing cancer in the uterus (cancer of the uterine lining, called endometrial cancer). For women who have not had a hysterectomy, an additional hormone called progesterone, or a synthetic version called progestin is given. Progesterone in combination with estrogen is called HRT. HRT works by replacing both estrogen and progesterone levels to mimic the levels that were in effect before menopause, and it reduces or eliminates the risk of endometrial cancer in women who have not had a hysterectomy. Like any medication, HRT can cause a number of unwanted side effects. Fortunately, most side effects are rare, and even the more common ones tend to disappear after the body adjusts to the hormones. |
| |
|
Nutrition
|
| |
| Calcium: The concept that osteoporosis is due, primarily, to calcium deficiency, particularly in the elderly was initially put forward as a counterproposal to Albright’s estrogen deficiency theory. Decreased calcium intake, impaired intestinal absorption of calcium due to aging or disease, as well as vitamin D deficiency can result in secondary hyperparathyroidism. The active hormonal form, 1,25 dihydroxy vitamin D (calcitriol), is not only necessary for optimal intestinal absorption of calcium and phosphorus, but also exerts a tonic inhibitory effect on parathyroid hormone (PTH) synthesis, so that there are dual pathways that can lead to secondary hyperparathyroidism53 Vitamin D deficiency and secondary hyperparathyroidism can contribute not only to accelerated bone loss and increasing fragility, but also to neuromuscular impairment that can increase the risk of falls54,55 Calcium is required to support bone growth, bone healing and maintain bone strength and is one aspect of treatment for osteoporosis. Other factors, such as protein, salt and vitamin D intake, exercise and exposure to sunlight, can all influence bone mineralization, making calcium intake one factor among many in the development of osteoporosis49. |
| |
| A meta-analysis of randomized controlled trials involving calcium and calcium plus vitamin D supported the use of high levels of calcium (1,200 mg or more) and vitamin D (800 IU or more), though outcomes varied depending on which measure was used to assess bone health (rates of fracture versus rates of bone loss).50 |
| |
|
Mode of Action:
|
| |
| After calcium is consumed, several nutrients, especially vitamin D, help the body absorb the calcium. |
| |
| The blood transports the calcium that is not needed for other body processes to the bones where it adds to the bone mass and is stored for when it is needed in the rest of the body. |
| |
| Sometimes a lack of calcium comes from not consuming enough in the diet or because the body is not absorbing enough into the blood. When this happens, calcium is removed from the bones into the blood to keep a constant level of calcium in the blood. Bones act as a storehouse for calcium, which is used by the body and replaced by the diet throughout a person's life. If enough calcium is not consumed, the body takes it from the bones. If more calcium is removed from the bones than is consumed in the diet, the bones become fragile and weak as a person gets older, leading to osteoporosis and fractures. |
| |
| Getting too much calcium is difficult. The body can absorb 2 g (2000 mg) of calcium a day, and anything more can be excreted in the urine, although too much calcium excretion through the kidneys can result in painful kidney stones. |
| |
|
Herbal Formulation:
|
| |
| The herbal formulation is a unique combination designed specifically to restore and maintain bone health. It not only builds elemental structure of bones but also has powerful anti – osteoporosis action. All the ingredients are carefully selected to meet all critical aspects of bone health. Some are rich source of natural calcium to fulfill body’s need of calcium during bone formation, some are having phytosterons which bolsters body’s healing power and improves muscular strength. It contains the following extracts of Cissus quadrangularis (Asthisanghat) 32%, Mucuna pruriens (Kapikachhu) 2%, Terminalia arjuna (Arjun) 2%, Asparagus racemosus (Shatavari) 2%, Ashwagandha (Withania somnifera) 2%, Vitex negundo (Nirgundi) 2%, Commiphora mukul (Guggulu) 1% and Excepients q.s. |
| |
| The principle action of each ingredient is as discussed below |
| |
|
Cissus quadrangularis (Asthisanghat):
|
| |
| It is a perennial plant of the grape family. Ayurveda mentions it as a tonic and, analgesic and prescribes its use to help heal broken bones, thus it’s called as asthisamharaka (that which prevents the destruction of bones). It has also been used to treat osteoporosis, asthma, cough, hemorrhoids, and gonorrhea. |
| |
| It contains a rich source of carotenoids, triterpenoids and ascorbic acid. Compounds that act as receptor antagonists of glucocorticoids have been found reducing the healing time of broken bones 30 to 50 % in clinical trials. It has also been used to treat obesity and associated oxidative stress. Its bactericidal effects on Helicobacter pylori hold promise as an effective treatment of gastric ulcers and preventative of stomach cancer in conjunction with NSAID therapy. |
| |
| The alcoholic extract of Cissus quadrangularis improved the rate of fracture healing13-14 and calcification process15 in experimental animals. The rate of bone fracture healing was markedly hastened in animals with alcohol free aqueous extract of the plant16 without affecting plasma biochemical parameters.17 |
| |
| It is reported to have a very good fracture healing effect. 13-18 |
| |
| |
|
Mucuna pruriens (Kapikachhu)
|
| |
| It’s a rich source of natural L-Dopa, precursor of Dopamine. The L Dopa free fraction of seed shows significant anti Parkinsonism activity as well. 19-20 Apart from this it contains many phytosterol compounds that help improves neuro-muscural coordination. |
| |
|
Terminalia arjuna (Arjun):
|
| |
| Terminalia arjuna is a medicinal tree of the genus Terminalia, widely used by Ayurvedic physicians for its curative properties in organic/functional heart problems including angina, hypertension and deposits in arteries. Its stem bark possesses glycosides, large quantities of flavonoids, tannins and minerals. Flavonoids have been detected to exert antioxidant, anti-inflammatory and lipid lowering effects while glycosides are cardiotonic, thus making it unique amongst currently used medicinal plants. It is also given in fractures and contusions with excessive ecchymosis. Its bark contains unusually high amount of calcium. Healing process in ulcerated, contused wounds and fractures are greatly enhanced by systemic administration of arjuna21 |
| |
|
Asparagus racemosus (Shatavari):
|
| |
| It is an evergreen creeper, native to India. Its root is rich in phytoestrogens, a group of natural compounds that have a chemical structure very similar to estrogen. Estrogen helps maintain the cardiovascular system and prevent osteoporosis in women especially in perimenoposal period. |
| |
|
Withania somnifera (Ashwagandha):
|
| |
| Its roots are internationally known as Indian ginseng, now scientifically proved as a good source of natural steroidal compounds. It exerts many positive steroidal actions but free from negative effects that synthetic steroids show. It has been found stimulating bone calcification and promising as a potential agent in the treatment of osteoporosis. |
| |
|
Vitex negundo (Nirgundi):
|
| |
| In Ayurvedic practice it is reputed as antiinflammatory and widely used in arthritic conditions. |
| |
|
Commiphora mukul (Guggulu):
|
| |
| It contains guggulu sterons, proven antiinflammatory agents, renowned for their anticholesterol, anti-arthritic actions. It helps reduce pain, stiffness and improve joint mobility in older patients with osteo-arthritis. |
| |
|
The Nutritional Value of the completed herbal formulation was also determined.67
|
| |
|
Total carbohydrate content:
|
| |
| The standard Anthrone method was used to check for the amount of Total Carbohydrate in the given sample. The sample to be tested was accurately weighed in boiling tubes and dissolved in 10 ml of 2.5 N HCl solution. It was then hydrolyzed by keeping it in a boiling water bath for 3 hours and cooled down to room temperature. It was then neutralized with solid Na2CO3 until the effervescence ceases and made up the volume to 100 ml with distilled water. It is then centrifuged and the supernatant was collected and two different aliquots were prepared. Glucose was used as a standard for the preparation of the standard graph with ranges of 0Rg - 200Rg concentration (0Rg served as a blank). Make up the volume to 2 ml with Distilled water then add 4 ml of anthrone reagent to all the tubes and heat for 10 minutes on a boiling water bath. Cool the tubes and their absorbance was read at 630 nm on a UV Spectrophotometer. |
| |
|
Protein Content:
|
| |
| The protein estimation was carried out by the Lowry’s method. Bovine serum albumin (BSA) was used as a standard for preparation of the standard graph with ranges from 0Rg - 250Rg concentration (0Rg served as a blank). The sample to be tested was weighed accurately and dissolved in distilled water and filter and use as the sample. Take different aliquotes and make up the volume with distilled water and add reagent C (Alkaline copper solution: Mix 50 ml of Reagent A and 1 ml of Reagent B) and incubate at room temperature for 10 minutes, after which 0.5 ml of Folin- Ciocalteau reagent was added and incubated at dark for 20 minutes and the absorbance was read at 660 nm on a UV Spectrophotometer. |
| |
| (Note: Reagent A- 2 % Sodium carbonate in 0.1 N NaOH, Reagent B- 0.1 % Na-K tarterate and 0.5 % CuSO4.) |
| |
|
Vitamin B2 (Riboflavin) content :
|
| |
| Accurately weigh the sample and dissolve it in 100 ml of 0.1 N H2SO4. Boil the sample for 30 minutes and allow it to cool down to room temperature and then add 5 ml of 2.5 M sodium acetate and incubate it at room temperature for 1 hour. Filter the solution and the filtrate is used as the sample for further analysis. Riboflavin is used as a standard for the preparation of standard graph which ranges from 0Rg - 250Rg concentration (0Rg served as a blank). Make up the volume to 2 ml with Distilled water. Add 1 ml of Glacial acetic acid followed by 0.5 ml 4% KMnO4. Incubate for 2 seconds and add 0.5 ml 30 % Hydrogen peroxide. Shake well and read the absorbance at 366 nm on a UV Spectrophotometer. |
| |
|
Vitamin B12 (Thiamine) content :
|
| |
| Accurately weigh the sample and dissolve it in 100 ml of 0.1 N HCl and mix well. Incubate it overnight. Filter the solution and the filtrate is used as the sample for further analysis. Thiamine is used as standard for preparation of the standard graph which ranges from 0Rg - 250Rg concentration (0Rg served as a blank). Make up the volume to 5 ml with Distilled water. Add 2.5 ml of Buffer solution having a pH 6.6 followed by 2.5 ml 4% Cyanogen bromide solution and shake well. Incubate for 30 minutes at room temperature and take the reading at 366 nm on a UV Spectrophotometer. |
| |
|
Vitamin C (Ascorbic acid) content :
|
| |
| Accurately weigh the sample and dissolve it in 75 ml of m- Phosphoric acid (m-PA) taken in SnCl2 solution and mix well. Filter the solution and the filtrate is used as the sample for further analysis. Ascorbic acid is used as the standard for preparation of standard graph which ranges from 0Rg - 250Rg concentration (0Rg served as a blank). Make up the volume to 2.5 ml with m-PA. Add 0.5 ml of 2% Dinitrophenyl hydrazine (DNPH). Incubate it for 1 hr at 50oC. Then add 2.5 ml of 85% sulphuric acid and take the reading at 540 nm on a UV Spectrophotometer. |
| |
|
Iron content:
|
| |
| Accurately weigh the sample and dissolve it in 50 ml of distilled water and mix well. Filter the solution and the filtrate is used as the sample for further analysis. Fe3+ is used as standard for preparation of standard graph which ranges from 0Rg - 250Rg concentration (0Rg served as a blank). Make up the volume to 2.5 ml with Distilled water. Add 0.5 ml of Hydroquinone solution followed by 2.5 ml of Acetate buffer having a pH of 5.0. Then add 0.5 ml of ?0.1% ? - ? di pyridine and incubate at room temperature for 30 minutes and take the reading at 540 nm on a UV Spectrophotometer. |
| |
|
Calcium content:
|
| |
| Accurately weigh the sample and dissolve it in a 150 ml conical flask containing 3 ml dilute HCl and 10 ml distill water. Boil for 10 minutes to dissolve the sample and cool down to room temperature. Dilute it with 50 ml of Distilled water. Titrate against 0.05 N disodium EDTA solution nearing the end point and then add 8 ml of 20 % NaOH solution with the addition of 0.1 g calcon mixture which acts as an indicator. Continue the titration till the end point is achieved. The percentage of Calcium is then calculated according to the formula below. |
| |
| % of Calcium = (Burette reading x Factor x Actual Normality of EDTA x 100)/ Weight of the sample x normality of EDTA |
| |
| Where Factor = 0.005004 |
| |
|
Cholesterol content :
|
| |
| Accurately weigh the sample and dissolve it in 50 ml of Isopropyl alcohol and mix well. Filter the solution and the filtrate is used as the sample for further analysis. Cholesterol is used as standard for preparation of the standard graph which ranges from 0Rg - 250Rg concentration (0Rg served as a blank). Make up the volume to 2 ml with Isopropyl alcohol. Add 1 ml of Fecl3 –acetic acid and add 2 ml of Conc. H2So4. Mix well and incubate at room temperature for 10 min and take the reading at 540 nm on a UV Spectrophotometer. |
| |
|
Fiber content :
|
| |
| Accurately weigh the sample and dissolve it in ether to remove the initial fat. Then boil it with 200 ml of Conc. sulphuric acid for 30 minutes. Filter and wash the boiling water until the washings are no longer acidic. Then boil it with 200 ml of 1.25% NaOH solution. Filter and wash with 25 ml of 1.25% H2SO4, three 50 ml portions of water and 25 ml alcohol. Remove the residue and transfer it to a previously weighed evaporating dish (W1). Dry the residue for 2 hr at 130°C, cool and weigh (W2). Ignite for 30 minutes at 600°C, cool and weigh (W3) again. |
| |
| The percentage of Crude fiber is calculated according to the formula below |
| |
| % of Crude fiber = (Loss in weight on ignition (W2 – W1) – (W3-W1) X 100)/ Weight of the sample |
| |
|
Microbial Analysis
|
| |
| Microbial analysis was carried out for all the three extracts as per procedure of Indian pharmacopoeia 2007 and WHO Guideline. It included total bacterial count, Total yeast and mould Count, Presence of Escherichia coli, Presence of Salmonella ebony, Presence of Pseudomonas aeruginosa, and Presence of Staphylococcus aureus. Pure culture of Escherichia coli (NCIM: 2065; ATCC: 8739), Salmonella ebony (NCIM: 2257 NCTC: 6017), Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6358). Obtained from NCIM Pune were used as control. The media used for the microbial limit test were of HiMedia Pvt. Ltd.22-23 The results are tabulated in Table 2. |
| |
|
Heavy Metal Analysis
|
| |
| Accurately weigh 2 g of the sample in a kjendal flask. An acid mixture of HNO3:HClO4 (4:1) was added in the flask and heated continuously till the solution becomes colorless or a white fumes ceases from the flask. The sample was then transferred to a 50 ml volumetric flask and volume was made up with distilled water. A reagent blank was synchronously prepared accordingly to the above procedure. The standards of Lead (Pb), Cadmium (Cd), Arsenic (As) and Mercury (Hg) were prepared and calibration curve developed for each of them. The sample were analyzed for the presence of Pb, Cd, As, and Hg using atomic absorbance spectrophotometer SHIMADZU AAS 6300 24 |
| |
Discussion:
|
| |
| The herbal formulation is formulated in such a way so as to aid in Osteoporosis. All the ingredients were carefully selected to meet all critical aspects of bone health. Some are rich source of natural calcium to fulfill body’s need of calcium during bone formation, some are having phytosterons which bolsters body’s healing power and improves muscular strength. The Pharmacological action aids in anti-osteoporotic, promotes calcification of bone and increases bone mass density. No side effects and drug to drug interaction were reported as well. |
| |
Conclusion:
|
| |
| The herbal formulation is thus an active source of Calcium and also contains many other ingredients that can be taken by person of any age group to prevent Osteoporosis. |
| |
|
Acknowledgement:
|
| |
| We are highly thankful to the management of VASU HEALTHCARE PVT LTD for providing us this opportunity in carrying out the research work. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
|
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |









