Ibegbu O Augustine1*, Okechukwu N. Gertrude1, Eze Martin1, Uchewa O. Obinna1, Ezemagu K. Uchenna1, Egwu A. Ogugua1, Sarah Al-Rashed2, Ibe Usman Michael3 and Saber Batiha4
1 Department of Anatomy, University of Alex Ekwueme Federal, Ebonyi State, Nigeria
2 Department of Botany and Microbiology, University of King Saud, Riyadh 11451, Saudi Arabia
3 Department of Anatomy, University of Kampala International, Bushenyi, Uganda
4 Department of Pharmacology and Therapeutics, University of Damanhour, AlBeheira, Egypt
*Corresponding Author:
Augustine O. Ibegbu
Department of Anatomy, University of Alex Ekwueme Federal, Ebonyi State, Nigeria,
Tel:+2348032188042;
E-mail: austine.ibegbu@funai.edu.ng
Received Date: June 22, 2021;Accepted Date: July 07, 2021;Published Date: July 14, 2021
Citation: Augustine IO, Gertrude ON, Martin E, Obinna UO, Uchenna EK, et al. (2021) Cerebellar and Hypocampal Changes Induced by Lead in Wistar Rats: The Role of Ocimium gratissimium Leaves Extract. J Bio Med Sci Vol.10 No.3:56.
Keywords
Brain barrier; Ocimum gratissimum; Wistar rats; Cerebellum; Hippocampus; Histology
Introduction
Lead poisoning is caused by increased levels of lead in the body’s blood level [1]. One of the primary and important targets for lead is the nervous system [1,2]. Even at low dose, lead is likely to penetrate into the brain by changing the working competence of the Blood–Brain Barrier (BBB) and this is easier in young children because their Blood–Brain Barrier (BBB) is not fully developed [3]. Increased lead poisoning is an influential factor in brain damage, mental inefficient and intense behavioral disorders as well as anemia, neuromuscular weakness as well as coma [4]. This increase has been linked to the rapid rising level of chemicals in the environment, particularly lead, which has well known hazardous effects [5,6] that can be toxic when introduced into the human and animal bodies by ingestion or inhalation [7,8]. Several studies have revealed the role of lead in hypertension, reduced renal function, decline cognitive function and adverse reproductive impact even in blood lead levels far below 25 μg/dL [9,10].
Acute and sub-acute effects of lead are caused by relatively large doses over a short period (days to months). Smaller amounts of lead taken over long period may lead to lowered sex drive, decreased fertility, miscarriages, premature births, learning impairments and increase aggression [11,12]. It is a known neuro-toxicant and also leads to cognitive malfunction [7,9,11].
The main target for lead toxicity is the central nervous system as such, the brain is the organ most studied in lead toxicity with symptoms of dullness, forgetfulness, irritability, poor attention span, headache, fatigue, impotence, dizziness and depression [12,13]. Human and animal populations throughout the world are exposed on daily basis to low levels of environmental contaminants [14,15].The human race is worried by increasing load of environmental contaminants, affecting not just health and behaviour, but finally survival of the species itself. Among these chemicals, lead is one of the most hazardous to living matter [16]. This metal is primarily found in leaded gasoline [17,18]. Automobile emissions have been an important source of lead exposure for urban residents, especially in areas with congested traffic [15]. The main source of adult human exposure is food, which is known to account for over 60% of blood levels; air inhalation accounts for approximately 30% and water of 10% [19]. Lead is highly toxic and can disrupt the body’s neurological, biological and cognitive functions [20]. Lead has been reported to induce cellular damage in the cerebellum of adult wistar rats and it was also observed that ascorbic acid has ameliorative effect on the lead induced cellular damages in the cerebellum of adult Wistar rats [21]. Many research groups have reported that lead could cause apoptosis in a number of experimental systems, including rat brain, rat and mouse retinal rod cells, Cerebellar neurons and PC12 neuronal cells [13,14,22-24]. The effects of lead on apoptosis in hippocampus have previously been reported [13,21].
The consumption of varieties of local herbs and vegetables by man add significantly to the improvement of human health, in terms of prevention and treatment of diseases that may arrive by rising level of environmental chemicals such as lead and mercury, because plants have long served as a useful and natural source of therapeutic agents [25]. The activities of these curative plants are evaluated by their chemical components. This is a tropical plant that belongs to the family of Labiatae and is a home grown shrub used mainly as spices for cooking delicacies due to its unique aromatic taste [25,26]. The plant is a perennial plant that is common in Asia and Africa. African Countries like Nigeria, Ghana and Cameroun often use it for both nutritional and medicinal purposes [26-28].The aim of the present study is to evaluate the effect of Ocimum gratissimum leaf on Lead induced changes in the cerebellum and hippocampus of adult Wistar rats.
Materials and Methods
Preparation of aqueous extract
Ocimum gratissimum leaves were purchased from a local farm in Abakaliki Ebonyi State, Nigeria, washed and air-dried for 14 days before grinding them into powder. Aqueous infusion was done by mixing a calculated volume of distilled water and powdered sample. The mixture was allowed to stand for 30 minutes before filtration. It was then centrifuged at about 3000 xg for 5 min and the supernatant collected. The supernatant was cleared of particles by suction filtration using Whitman no 1. Filter paper and cellulose filter paper. The extract was subsequently concentrated to dryness in vacuum at 10o C and 10% w/w using a rotary evaporator and stored in a desiccator. At the end a dark greenish extract was obtained and used.
Lead acetate and ascorbic acid
Five hundred (500) g of Lead acetate of 99.5%-100.5% purity with product No Loo5112 and Batch No.CSA200112 was obtained from Histology Laboratory, Department of Anatomy, Federal University Ndufu Alike Ikwo, Ebonyi State. Then 20% amounting to 120 mg/kg body weight of the LD50 of 600 mg/kg body weight of the lead acetate was dissolved in distilled water and used for the experiment. Vitamin C was purchased from Michelle Laboratories Ltd, Enugu, Nigeria with NAFDAC No. 04-0452 and Batch No. VC306.
Experimental procedure and groupings
Ethical clearance was obtained from the University Ethics and Animal Handling Committee with approval Number AE-FUNAI/ EAHC/12/17. Thirty five (35) adult Wistar rats used in this study were purchased from the Animal house of the University and kept in the same animal house. The Wistar rats were randomly divided into seven groups of 5 rats per group according to the methods of [29]. Control group (Group 1) animals received distilled water. Group 2 were given 20% of the LD50 of lead acetate amounting to 120 mg/kg body weight, Group 3 animals received 30% of the LD50 of Ocimum gratissimum at 375 mg/kg, Group 4 received 30% of the LD50 of Ocimum gratissimum and 20% of LD50 of lead acetate amounting to 375 mg/kg of Ocimum gratissimum and 120 mg/kg of lead acetate. While Group 5 animals received 60% of LD50 of the Ocimum gratissimum and 20% of LD50 of lead acetate at 750 mg/kg Ocimum gratissimum and 120 mg/kg of lead acetate, Group 6 animals received 60% the LD50 of OG for two weeks and then 20% of the LD50 of Lead for one week at 375 mg/kg of Ocimum gratissimum for 2 weeks then 120 mg/kg of lead acetate for 1 week and Group 7 received 10% of the LD50 of Vitamin C and 20% of LD50 of Lead at 1190 mg/kg of Vitamin C and 120 mg/kg of lead acetate. The administration of the lead and the Ocimum gratissimum extract were carried out by oral gavage every day for a period of twentyone (21) days.
Animal sacrifice
At the end of the administration, all the animals were sacrificed via cervical dislocation and the heads of the animals decapitated and immersed in 10% formal saline for 2 days. After which the brains were removed and the cerebellum and hippocampus were carefully removed and fixed in 10% formal saline. The tissues were processed and serial paraffin sections of 5 μm were made and stained using Haematoxylin and Eosin (H and E) method.
Statistical analysis
All the results were analyzed using Statistical Package for Social Scientist (SPSS version 20). The Data was expressed as mean ± SEM and were analyzed. Statistical significance between the means was analyzed using one-way analysis of variance (ANOVA) and P-value ≤ 0.05 was considered statistically significant.
Results
During the period of administration, the animals were observed to be using their forelimbs to scratch their mouth area. It was observed that on lead acetate administration, there was reduction in the physical activities and presence watery-black stool in the animals. There was decline in food consumption due to loss of appetite for food. Table 1 below shows that there was a gradual increase in the mean body weight of the animals in Control. A decrease in body weight was recorded in lead group (Groups 2) when compared to the control. There was also a gradual increase in weight in groups 6 and 7 (Treated with 30% of the Ocimum gratissimum for two weeks and 20% of the LD50 of lead for one week, and 10% of the LD50 of Vitamin C and 205 of the LD50 of Lead) showing that there is an ameliorative effect of Ocimum gratissimum and Vitamin C.
| Groups |
Initial weight (g) |
Final weight (g) |
Weight change (g) |
(%) weight change |
| 1 |
89.90 ± |
114.5 |
± |
24.6 ± 2.94 |
27.36 |
| |
15.93 |
12.99 |
|
|
|
| 2 |
159.1 ± |
153.9 ± |
5.2 ± 17.78 |
3.27 |
| |
25.06 |
42.84 |
|
|
| 3 |
127.4 ± |
151.9 ± |
24.5 ± 16.15 |
19.23 |
| |
33.84 |
49.99 |
|
|
| 4 |
108.7 ± |
137.7 ± |
29 ± 4.46 |
26.68 |
| |
24.00 |
19.34 |
|
|
| 5 |
189.1 ± |
201.4 ± |
12.3 ± 12.84 |
6.5 |
| |
33.11 |
20.27 |
|
|
| 6 |
110.5 |
± |
151.2 ± |
40.7 ± 18.17 |
36.83 |
| |
6.816 |
|
24.99 |
|
|
| 7 |
118.7 |
± |
145.0 ± |
26.3 ± 3.44 |
22.17 |
| |
15.23 |
|
11.79 |
|
|
The values are expressed as Mean ± STD; N=No. of animals
Table 1: Weight change comparison of Wistar rats at initial and final day of treatment.
Histological studies
The results from microscopical examination showed intriguing histological changes in the tissues studied. The cerebellum of the Control group (Group 1) showed normal cyto-architecture with well-defined three layers such as outer Molecular, inner Granular and intermediate Purkinje layers as shown in Figure 1A. While the cerebellum of lead only group showed marked degeneration and vacuolation of neuronal cells with much of the Purkinje layer as seen in Figure 1B. Group 3 showed normal histological structures similar to that of the Control see Figure 1C. Group 4 showed near normal purkinje cells with little degeneration while Groups 5, 6 and 7 showed normal cytoarchitecture of the cerebellum similar to that shown in Figure 1D. Figures 2A-2D result presents normal hippocampus in the Control with normal pyramidal cells as shown in while the lead only group showed severe degeneration and disruption of the hippocampus with loss of its fundamental histological features mainly the Pyramidal layer and pyramidal cells as in Figures 3A and 3B. The pyramidal cells appeared smaller than the cells in the Control Group (Group 1). The hippocampus of the rat in Group 3 showed normal cyto-architecture of the hippocampus similar to that of the Control group (Figures 3A- 3C). The hippocampus of Group 4 with pyramidal layer and normal pyramidal cells and some degenerated pyramidal cells as compared with the Control group (Figures 3A and 3D). However, , showed the hippocampus of Groups 5, 6 and 7 with scattered Pyramidal cells which was similar to that of the Control group in Figures 4A-4D.
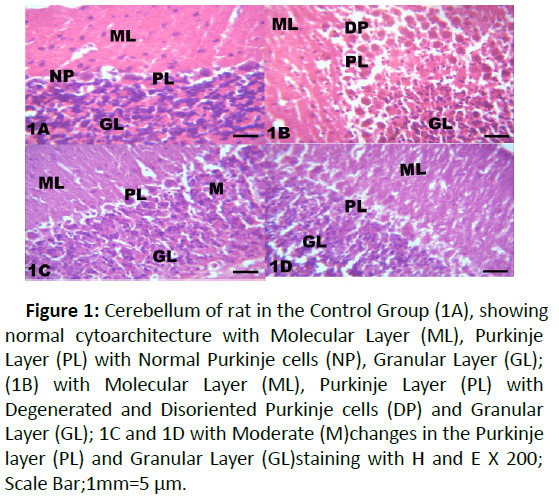
Figure 1:Cerebellum of rat in the Control Group (1A), showing normal cytoarchitecture with Molecular Layer (ML), Purkinje Layer (PL) with Normal Purkinje cells (NP), Granular Layer (GL); (1B) with Molecular Layer (ML), Purkinje Layer (PL) with Degenerated and Disoriented Purkinje cells (DP) and Granular Layer (GL); 1C and 1D with Moderate (M)changes in the Purkinje layer (PL) and Granular Layer (GL)staining with H and E X 200; Scale Bar;1mm=5 μm.
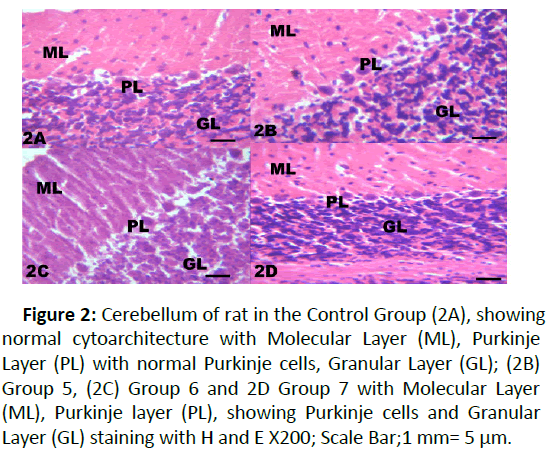
Figure 2: Cerebellum of rat in the Control Group (2A), showing normal cytoarchitecture with Molecular Layer (ML), Purkinje Layer (PL) with normal Purkinje cells, Granular Layer (GL); (2B) Group 5, (2C) Group 6 and 2D Group 7 with Molecular Layer (ML), Purkinje layer (PL), showing Purkinje cells and Granular Layer (GL) staining with H and E X200; Scale Bar;1 mm= 5 μm.
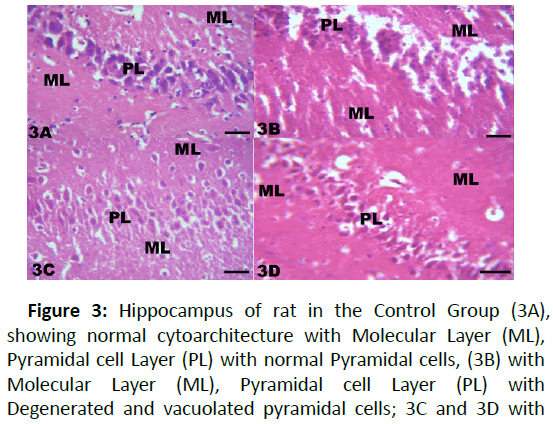
Figure 3: Hippocampus of rat in the Control Group (3A), showing normal cytoarchitecture with Molecular Layer (ML), Pyramidal cell Layer (PL) with normal Pyramidal cells, (3B) with Molecular Layer (ML), Pyramidal cell Layer (PL) with Degenerated and vacuolated pyramidal cells; 3C and 3D with moderate changes in the Pyramidal Layer (PL); staining with H and E: X200; Scale bar;1 mm=5 μm.
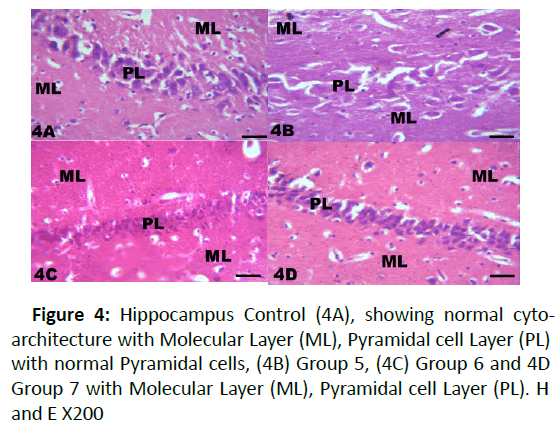
Figure 4: Hippocampus Control (4A), showing normal cytoarchitecture with Molecular Layer (ML), Pyramidal cell Layer (PL) with normal Pyramidal cells, (4B) Group 5, (4C) Group 6 and 4D Group 7 with Molecular Layer (ML), Pyramidal cell Layer (PL). H and E X200
Discussion
It has been reported that rats that are exposed to heavy metals such as mercury, Cadmium, Arsenic and Lead usually results in reduction in the body weight [30]. The present study was carried out to study the effect Ocimum gratissimum on lead induced changes on the cerebellum and hippocampus of adult Wistar rats and the result showed that there was reduction in the mean body weight of rats in the lead treated Group when compared with rats in the Control Group. The reduction in the mean body weight with exposure to lead could be as result of loss of appetite oro xidative stress linked ot heavy metals [9,31,32]. This is in agreement with other studies on the effect of lead on animal body weight [33,34]. The result of the present study showed that rats treated with lead acetate and Ocimum gratissimum or Vitamin C showed slight increase in the body weight which when compared with the rats in the Control Group was not significant. The slight increase in the mean body weight in treated Groups may be attributed to the antioxidant property of Ocimum gratissimum which could have mopped up the free radicals generated by lead administration to the rats.
Several studies have revealed that lead exposure produces neurological damages and behavioral disruptions experimental animals which may result in behavioral alternation, learning and memory loss [33,35]. Histological examinations of Cerebellar and hippocampal sections in our study showed normal cytoarchitecture of the cerebellum and hippocampus in the Control while lead treated showed degeneration and disruption of the cellular layers of cerebellum and hippocampus. There was near normal cyto-architecture of the cerebellum with relatively little alteration gorup treated withO cimum gratissimum. These changes include disruption of the layers especially Purkinje cell layer, loss of cellular architecture of Purkinje cells which could ultimately interfere with the activities and functions of the cerebellum and other motor functions like maintenance of equilibrium, loss of grasping, loss of fine movement, and loss of regulation omf uscle tone. These morphological changes observed in the tissues were supported by previous neuropathological findings [32-35].
In the hippocampal sections of the Control Group rats, there were no sign of histological changes with normal pyramidal layer while the histological sections of the rats in lead acetate only treated Group 2, showed a number of histological changes ranging from degeneration and reduction in the number of pyramidal cells. This entails that the activity of the hippocampus in memory formation and learning will be altered and the role of the hippocampus that involved storage and retrieval of information will be lost. According to, lead resulted in deleterious effects on the tissues of the hippocampus by distorting the general histological forms and cellular integrity while Moringa oleifera extract ameliorated the severity of the lead toxicity [36]. Meanwhile, the histological sections of the rats treated with lead acetate, aqueous Ocimum gratissimum extract and Vitamin C, showed preserved normal histological form of the hippocampus which was in agreement with the findings [37,38]. Pyramidal cells were prominent, showing normal and healthy morphology. This implied that aqueous Ocimum gratissimum extract has promising effects against lead induced toxicity in Wistar rats.
Conclusion
It can be concluded from the present study that Lead acetate induced changes in the body weight of the adult Wistar rats and induced histological changes ranging from degeneration of the nerve cells and distortion of the cellular layers of the cerebellum and hippocampus in the lead treated groups while the administration of Ocimum gratissimum extract showed alleviation and reduction of the effects induced by lead in the treated groups.
38849
References
- Patrick L (2006) Lead toxicity a review of the literature part: exposure, evaluation and treatment. Altern Med Rev 11:2-22.
- Sujatha K, Srilatha C, Anjaneyulu Y, Amaravathi (2011) Lead acetate induced neurotoxicity in Wistar albino rats: A pathological, immunological, and ultrastructural studies. J Pharm Bio Sci 2:459-462.
- Goldstein GW (1996) Brain capillaries: a target for inorganic lead> poisoning. Neurotoxicology 5:167-175.
- Surana V, Jain D (2010) Protective effect of Ocimum gratissimum against carbon induced hepatic damage in rats. Pharmacology online 2: 1111-1119.
- Cristiana M, Murbach F, M´arcia OM, Mirtes C (2006) Effects of seasonal variation on the central nervous system activity of Ocimumgratissimuml. essential oil. J Ethnopharmacol 105; 161-166.
- Garcia MTA, Gonzalez ELM (2008) Toxic effects of perinatal lead exposure on the brain of rats: Involvementof oxidative stress and the beneficial role of antioxidants. Food Chem Toxicol 46:2089-2095.
- Finkelstein Y, Markowitz ME, Rosen JF (1998) Low-level lead- induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev 27:168-176.
- Barbhuiya SSK, Chakraborty S and Sengupta M (2013) Studies of lead toxicity on inflammatory damage and innate immune functions in testicular macrophages of male Swiss albino mice. Modern Research in Inflammation 2:75-81.
- Buck M, Chojkier M (1996) Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15:1753-1765.
- Kosnett MJ (2009) Health effects of low dose lead exposure in adults and children, and preventable risk posed by the consumption of game meat harvested with lead ammunition. Med 1:24-33.
- Watson RT, Fuller M, Pokras M (2008) Hunt Ingestion of Lead from Spent Ammunition: Implications for Wildlife and Humans. J Ornithol 122:198-199.
- Pokras MA, Kneeland MR (2009) Understanding lead uptake and effects across species lines: A conservation medicine approach.
- Watson RT, Fuller M, Pokras M, Hunt WG (2008) Ingestion of Lead from Spent Ammunition: Implications for Wildlife and Humans.
- Waggas MA (2012) Grape Seed Extract (Vitisvinifera) Alleviate Neurotoxicity and Hepatotoxicity Induced by Lead Acetate in Male Albino Rats. J Behavioral and Brain Science 2:176-184.
- He L, Perkins GA, Poblenz AT, Harris JB, Hung M, et al. (2003) Bcl- xL overexpression blocks baxmediated mitochondrial contact site formation and apoptosis in rod photoreceptors of lead-exposed mice. Natl Acad Sci 100:1022-1027.
- He L, Poblenz AT, Medrano CJ, Fox DA (2000) Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J Biol Chem 275:12175-12184.
- Bolognesi C, Morasso G (2000) Genotoxicity of pesticides: Potential risk for consumers. Trends in Food Science and Technology 11:182-187.
- Alissa EM, Ferns GA (2011) Heavy metal poisoning andcardiovascular disease.
- Srianujata S (1997) Lead the toxic mental to stay with human. J Toxicological Sciences 23:237-240.
- Tong S, Von Schirnding Y, Prapamontol T (2000) Environmental lead exposure: A public health problem of global dimensions. Bulletin WHO 78:1068-1077.
- John H, Cheryl H, Richard S, Christine S (1991) Toxics A to Z A guide to everyday pollution hards.
- World Health Organization (WHO) (2016) Lead poisoning and health. WHO Bulletin Archived from the original.
- Musa SA,Musa IO,Hamman WO,IbegbuA O,Umana UE (2012) Preventive activity of ascorbic acid on lead acetate induced Cerebellar damage in adult Wistar rats. Medical and Health Science Journal 13:99-104.
- Tavakoli-Nezhad M, Barron AJ, Pitts(D2K001) Postnatal Inorganic Lead Exposure Decreases the Number of Spontaneously Active
- Oberto A, Marks N, Evans HL, GuidottiA (1996) Lead promotes apoptosis in newborn rat cerebellar neurons: pathologicalimplications. J Pharmacol Exp Ther 279:435-442.
- Sharifi AM, Mousavi SH (2008) Studying the Effects of lead on DNA fragmentation and proapoptoticBax and antiapoptotic Bcl-2 protein expression in PC12 cells. Toxicol Mech Methods 18:75-79.
- Adebolu TT, Oladimeji SA (2005) Antimicrobial activity of leaf extracts of Ocimumgratissimumon selected diarrhoea causing bacteria in Southwestern Nigeria. Afr J Biotechnol 4:682-684.
- Eboh DEO, Ekundina VO (2012) Histomophological effect ofchronic administration of Occimum gratissimum on some organsof adult wistar rats. Nigerian J Clini Sci Res 12:69-75.
- Akinmoladun AC, Ibukun EO, Emmanuel A, Obutor EM, Farombi EO (2007) Phytochemical constituents and antioxidant activity of extract from the leaves of Ocimumgratissimum. Sci Res Essay 2:163-166.
- Akinyemi KO, Medle UE, Smith ST, Oyefolu AO, Coker AO (2004) Screening of some medicinal plants for antisalmonella activity. J herbpharmacother 5:45-60.
- Okechukwu GN, Ezor E, Finbarrs-Bello E, Ebube EB, Uzomba GC et al.(2019) Effects of Aqueous Extract of OcimumgratissimumLeaves and Vitamin C on Lead Acetate-induced Changes in the Thymus of Adult Wistar Rats. IJBCRR 26:1-9.
- Nwokocha CR, Owu DU, Ufearo CS,Iwuala MO (2011) Comparative study on the efficacy of Garcinia kola in reducing some heavy metal accumulation in liver of Wistarrats. J Ethnopharmacology 135:488-491.
- Klaassen CD (2001) Heavy metals and heavy-metal antagonists In: Goodman and Gilman’s.
- 32 Yeh YC,Liu TJ,Wang LC,Lee HW,Ting CT (2009)A standardized extract of Ginkgobilobasuppresses doxorubicin-induced oxidativestress and p53-mediated mitochondrial apoptosis in at testes. Br JPharmacol 156:48-61.
- Amjad Z, Muhammad ZI, Amir AS (2013) Lead-lnduced reduction in body and kidney weight of Wistar albino rats ameliorated by Ginkgo biloba extract. Biochem Physiol 2:113-116.
- Hamza GA, Ibegbu AO, Buraimoh A (2017) Evaluation of the effects of aqueous garlic extract on lead-induced changes on cerebellum of Wistar rats. African Journal of Cellular Pathology 8:9-14.
- Owolabi J,Williams F,Fabiyi O (2014) Evaluation of Moringa’s Effects Against Lead- Induced Disruption of the Hippocampus in Animal Models. World J Life Sci and Medical Research 3:39-45.









